Guest commentary from Jim Acker (GSFC/Adnet)
Research on the ocean carbonate cycle published in 2019 supports results from the 1980s – in contrast to many papers published since then.
During my graduate school education and research program in the 1980s, conducted at the Department of Marine Science (now the College of Oceanography) of the University of South Florida in St. Petersburg, I participated in research on the production (biogenic calcification) and fate of calcium carbonate (CaCO3) in the open waters of the northern Pacific ocean. There were two primary aspects of this research: one, to measure the sinking flux of biogenic materials in the water column of the Pacific Ocean, and two, to measure the dissolution rates of aragonite, a CaCO3 crystal structure (polymorph) formed by pteropods, under in situ conditions of temperature, pressure, and seawater chemistry.
Prior to this period, most estimates of the sinking flux of particles, organisms, and pieces of organisms had been made through the use of bottom-moored sediment traps deployed for weeks or months before recovery of their collected contents. Our research group, instead, used free-floating sediment traps attached to floats at the surface and deployed for very short periods of time, 24-36 hours. A primary reason for the free-floating method was that the sediment traps would move with the water currents at their deployment depth, reducing potential effects of current flow that might happen around a stationary moored trap. The free-floating traps were much bigger than moored traps, consisting of fiberglass cones about 4 meters high, with a capture area about a meter across.
To sum up, our research results were remarkably at odds with prior results. We found that pteropods dominated the sinking flux of biogenic CaCO3 at all depths deployed (100, 400, 900, and 2100 meters). The organisms to which the main mass of sinking CaCO3 had been previously attributed, sand-grain size foraminifera and microscopic coccolithophores (both composed of calcite), were minor constituents in our trap collections.
Aragonite is important because this crystal form is more soluble in seawater than calcite. So if the aragonite flux was much larger than the calcite flux, the standard concepts at that time regarding the formation and particularly dissolution of CaCO3 in the water column would be markedly changed. This is vital to characterize, because the absorption of increasing amounts of anthropogenic carbon dioxide (CO2) from the atmosphere causes ocean acidification, and this ongoing process would dissolve considerably more aragonite than calcite as the chemistry of the water column changed. My dissolution experiments also showed the likelihood of substantial dissolution of pteropod shells while they were sinking, especially in the north Pacific Ocean where corrosive waters occur at shallow depths (only hundreds of meters), compared to a couple of kilometers deep in the Atlantic. Up until then, most of the dissolution of biogenic CaCO3 had been thought to occur after the sinking particles reached the seafloor – out of reach of the immediate effects of ocean acidification.
So our results were published (Betzer et al. 1984 and Byrne et al. 1984), and were even accompanied by a commentary piece in Nature (“Surprise from the shallows” by Michael Whitfield of the Plymouth Marine Laboratory), in which he discussed how our results were fairly revolutionary. We found support for our results from papers by Robert Berner of Yale, in which he had postulated the aragonitic pteropods were likely to be an important component of the sinking flux of biogenic CaCO3. (Berner, R. A., 1977, Berner and Honjo 1981).
Thus, after getting my Ph.D., due to the historic impact of these papers in overturning cherished tenets of chemical oceanography, I had to consider offers from several prestigious oceanographic institutions. So I moved on to academic fame and tenured fortune.
(Not exactly.)
About a year after our papers were published, a paper appeared criticizing our results (Harbison and Gilmer 1986), indicating that the biological behavior of pteropods, which are free-swimming zooplankton, could have caused “overtrapping” of the organisms. In addition to the possibility that they just swam into the trap, pteropods feed by creating a net of mucus that captures organic particles, and they reel in the net to ingest the particles. When disturbed, pteropods cut the net and sink. So the paper suggested that if they were disturbed by a trap floating underneath them, they would cut the net and sink into it. Thus, the derived suggestion for doing sediment trap collections of sinking biogenic CaCO3 was to basically toss out any possible swimmers and not measure them at all. With few exceptions, this has been the unwritten but accepted protocol of sediment trap measurements of sinking CaCO3 flux since the 1980s. Other papers generally supported these results, so estimates of both planktonic calcification and the global sinking CaCO3 flux were based on the mass of foraminifera and coccolithophores.
However, during the period between then and now, there were indications that this might not be quite right. In particular, accurate measurements of the alkalinity (mainly bicarbonate and carbonate ion concentration, and a little borate ion) of seawater globally, from depth to surface, indicated that there was probably a good amount of CaCO3 dissolution taking place at shallow depths, even shallower than the saturation horizons for calcite and aragonite. This was peculiar both because of calcite’s resistance to dissolution, and because estimates of planktonic mass just didn’t provide a lot of CaCO3. And it turns out that some pteropod species, especially the thin-shelled polar species Limacina helicina, are important prey species in the polar oceans, including for fish like herring and larval salmon. If there is enough of them to keep the fish fed, one would think that there is enough of them to have some effect on seawater chemistry when they sink and dissolve.

A couple of weeks ago, I was responding to a tweet about pteropods with a don’t-you-forget-about-us tweet citing our 1984 papers. I happened to look at the online version of the paper, which due to the wonders of technology now provided links to recently published papers citing it. I noticed that one of these papers, Buitenhuis et al., was published in 2019, so I took a look, expecting the citation of our work would be a polite afterthought. The “Plain Language Summary” is shown below.
“We show that pteropods contribute at least 33% to export of CaCO3 at 100m and up to 89% to pelagic calcification. This is in line with results by Betzer et al., 1984 and Byrne et al., 1984, and contradicts most of the work that has been published since then, which has tended to argue for the dominance of either coccolithophores or foraminifers. Pteropods precipitate CaCO3 in the crystal form of aragonite. This is more soluble than calcite, which is produced by coccolithophores and pelagic foraminifers. Thus, the ocean alkalinity cycle and associated buffer capacity for CO2 could be more sensitive to rising CO2 than has been suggested by existing Earth System Models, which only represent calcite.”
Buitenhuis et al. (2019)
To put it mildly, I was intrigued. Actually, I jumped up and down in the hallway while pumping my fists and shouting. Fortunately it was late in the workday. I immediately emailed my co-major professor Robert Byrne, and in the grand tradition of triumphantly pithy statements, stated “We wuz RIGHT!” (He promised to tell my other major professor, Peter Betzer.)
So, what was done in this paper? Erik Buitenhuis of the Tyndall Centre for Climate Change Research and the University of East Anglia, and his co-authors, added the three main groups of planktonic calcifiers to a sophisticated biogeochemical model of the oceans. The model runs provided estimates of both the planktonic mass and the sinking fluxes resulting from it. So pteropods, essentially zeroed out before this, are suddenly responsible for nearly 90% of the total amount of open water CaCO3 formation the global surface waters. And more importantly, the sinking flux, where they were also zeroed out, now requires about 1/3 aragonite and 2/3 calcite, with aragonite the more soluble, and thus more sensitive, crystal form.
Three combined constraints – observations of pteropod biomass concentrations, pteropod growth rates, and aragonite production rates – meant that the model requires much more planktonic calcification overall than previous estimates, and also requires substantial dissolution shallower than the saturation horizon, where seawater is supersaturated with respect to CaCO3. Because it is undersaturated waters that are corrosive, you may wonder how that can happen. There are a couple of pathways – ingested prey can be dissolved in the guts of the predator, resulting in the excretion of highly alkaline fecal material, or when the organisms die, bacterial respiration will acidify the organic matter of the soft parts, essentially dissolving the shells from within. Also, pteropods vertically migrate, and in the polar Pacific, they can vertically migrate into undersaturated water, potentially causing shell damage such as that seen in Figure 2.
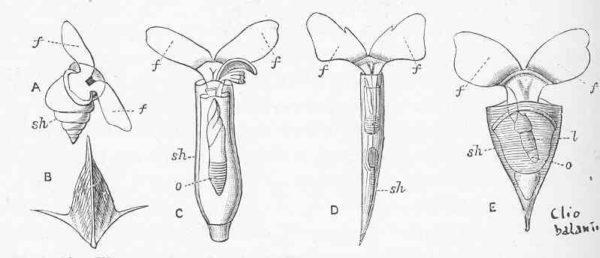
However, there is one other aspect to this whole story. While not accounting entirely for the alkalinity distribution in the oceans, measurements can get much closer to the models if pteropods are not excluded from sediment trap collections! There is support for this altered protocol, cited in the paper. I will also add, anecdotally, that we collected empty pteropod shells at the deeper depths of our trap deployments, including a pristine Clio pyramidata shell. This shell, which looks like a spaceship designed by Santiago Calatrava, has the same mass of a lot of foraminifera and a whole lot of coccolithophores. There was no reason, in my mind, to exclude the mass of empty pteropod shells from quantitative assessments.
Buitenhuis is currently working out the implications of this publication, particularly the impact on the oceanic alkalinity cycle and the global ocean sink for CO2. A greater mass of aragonite in surface waters and in the sinking flux could substantially change our understanding of the dynamics of the oceanic response to the effects of the absorbing more CO2. In fact, the pteropods may have bought us a little more time to react, if in the prophetic words of Michael Whitfield they “might act as a more rapid and effective sink for fossil-fuel CO2 than had been thought possible when attention was focussed on the dissolution of calcite from the ocean sediments”. Feedbacks of the changing alkalinity cycle can also affect the rate of ocean acidification, especially in shallower waters where acidification may detrimentally influence the life cycle of important seafood risotto constituents like crab and clams.
Which leaves us at the present. At the AGU Ocean Sciences meeting in San Diego, in February, there will be a couple of sessions about the carbonate cycle of the North Pacific Ocean. One of the chairpersons, Richard Feely of NOAA, was the principal investigator on the research cruises in the 1980s that I participated on (and a valued co-author, too). Though Erik Buitenhuis (who no longer flies due to his personal concerns about climate change) won’t be there, one of his co-authors, Nina Bednarsek, will be co-chairing these sessions and also giving a presentation prominently featuring pteropods, which have been the subject of much of her research.
I wish I could be there too. But thanks to these new research results, in essence, I think that my co-authors and I are already in attendance.
References
- P.R. Betzer, R.H. Byrne, J.G. Acker, C.S. Lewis, R.R. Jolley, and R.A. Feely, "The Oceanic Carbonate System: A Reassessment of Biogenic Controls", Science, vol. 226, pp. 1074-1077, 1984. http://dx.doi.org/10.1126/science.226.4678.1074
- R.H. Byrne, J.G. Acker, P.R. Betzer, R.A. Feely, and M.H. Cates, "Water column dissolution of aragonite in the Pacific Ocean", Nature, vol. 312, pp. 321-326, 1984. http://dx.doi.org/10.1038/312321a0
- R.A. Berner, "Sedimentation and Dissolution of Pteropods in the Ocean", The Fate of Fossil Fuel CO2 in the Oceans, pp. 243-260, 1977. http://dx.doi.org/10.1007/978-1-4899-5016-1_14
- R.A. Berner, and S. Honjo, "Pelagic Sedimentation of Aragonite: Its Geochemical Significance", Science, vol. 211, pp. 940-942, 1981. http://dx.doi.org/10.1126/science.211.4485.940
- G. Harbison, and R. Gilmer, "Effects of animal behavior on sediment trap collections: implications for the calculation of aragonite fluxes", Deep Sea Research Part A. Oceanographic Research Papers, vol. 33, pp. 1017-1024, 1986. http://dx.doi.org/10.1016/0198-0149(86)90027-0
- E.T. Buitenhuis, C. Le Quéré, N. Bednaršek, and R. Schiebel, "Large Contribution of Pteropods to Shallow CaCO3 Export", Global Biogeochemical Cycles, vol. 33, pp. 458-468, 2019. http://dx.doi.org/10.1029/2018GB006110
Thanks, most interesting.
Interesting stuff. Quantifying the different elements (rates, reservoirs, etc.) of the carbonate-silicate cycle is of especial interest to me, as I recently tried to model just that for a long-term Earth climate evolution model. Keep up the good work!
Sometimes unbelievable how sound theories are dismissed out of obviously flawed arguments. Any form of C that sinks to the seafloor is part of the carbon pump of the oceans and if it only reaches several hundred meters it’s still out of the system for a time and can still be metabolized by other living forms (e.g. heterotrophic prokariotes being eaten by tunicates wich eventually die and sink) and thus becomes part of the next step of the carbon pump. So any process that passes C down to deeper depths is part of the sedimentation chain. Even to image to cancel aragonite out of the equation is beyond me. It lives, has C content, it’s heavier than the surrounding water, it dies, it sinks it’s part of the C-pump and if its abundant it becomes an important part – its quite simple but then it becomes realy complicated, because many processes become thus important and not just one functional group of calcifiers which die and sink. But nice that the last years (decades) broke with many simplyfications e.g. incorportating marine snow. And if a fecal pellet does not reach the seafloor does not mean that the C will not be further processed and partly be passed further downwards.
I wonder how long it will take to admit that overfishing had a strong impact on the carbon pump (massively reduced biomass of standing stock, declining age structure, e.g. reduced individual lifetime fecal pellets production or drastically reduced mortality rate by taking the fish out of the system). How much C was one Potwale processing during its lifetime and shiting to deeper depths? I really don’t want to know the number of a single year. But still, it could be even negative (sedimentation rate of expired lifetime krill against eaten up krill could be a head to head race). But never saw a study exploring this aspect of the biological carbon pump or one citing this aspect.
Therefore, always adhere to the first rule contemporary Earth-System science: if you can not quantify something, doesn’t matter how big the possible impact and direction of recent observations, don’t include it at all in the models, otherwise, they could become wrong – the oxymoron of the model community ;)
Sheers and thanks for your important research and that it was acknowledged at least!
Jan
Could reducing the temperature of the Pacific mixed layer reduce the rate of solution of pteropod aragonite?
Thanks very much. We should have more biologists in the discussion of the unfolding biospheric catastrophe of man-made carbonic acid contamination (aka “climate change”).
A question comes to mind. Could we be in danger of compromising the protective shells of phytoplankton via carbonic acidification to such an extent that these single cell organisms could no longer produce the amount of oxygen they now do?
Perhaps we should add techniques that promote reproduction and stability of pteropods to fishing and trawling methods plus discharge of wastewater and runoff into coastal waters.
D. Shallin Busch et al found that survival of pteropods from Puget Sound increased with aragonite saturation; “Survival increased linearly with aragonite saturation state, though discrete treatment comparisons indicated that survival was similar in all but the lowest saturation state treatment.”
Washington State may attempt to save the local orca population by saving their prey – local populations of salmon – among other strategies like removing obstructions to salmon migration.
Recent reports in the news for Seattle of juvenile Dungeness crabs showing shell damage attributed to acidification don’t seem to be enough of a warning to people that there’s more to the threat of collapsing food webs than they think.
It’s been more than a decade since oyster hatcheries in Oregon and Washington had whole seed crops fail from acidification. Please note that they didn’t even figure out what was happening since they didn’t take pH readings prior to the failures. It took UW researchers to figure out what was happening.
Reducing the amount of atmospheric CO2 is the only answer but it’s been reported that some of the acidic seawater that harmed the oyster crops was upwelling waters which sank off the coast of Japan and Russia around fifty five years ago.
So even if we stop using fossil fuels there’s gonna be trouble for a long time afterwards.
It’s complicated but lower the temperature increases the solubility of gases like CO2. So does decreasing the salinity so more freshwater runoff and mixing adds more CO2. Washington State and all of BC and probably Alaska are getting snow melt earlier from climate warming.
It’s been decades since I studied this but less CO2 seems to be the only answer or accepting drastic changes to populations in the oceans.
An article by the Petrovskii group in (Bulletin of Mathematical Biology December 2015Volume 77, Issue 12, pp 2325 point out 70 % of the atmospheric oxygen is produced in the oceans by the photosynthetic activity of phytoplankton. Any disruption in the oxygen production from the ocean could affect the levels in the atmosphere world wide. Lower levels of oxygen could be detrimental to larger terrestrial organisms.
Charles Hollohan said “So even if we stop using fossil fuels there’s gonna be trouble for a long time afterwards.”
Yeah. True in multiple dimensions, clearly. Just less worse trouble than if we don’t.
The ocean may produce 70% of the world’s oxygen, but the atmospheric imbalance is maintained by burying the organic matter. Otherwise, when plankton respire they will just consume the oxygen when producing CO2. Burning of fossil fuels leaves a detectable decrease in oxygen:
http://scrippso2.ucsd.edu/
However, it is in the parts per million range, with atmospheric O2 > 20% by volume.
World hydro-electric dams destroying the ocean carbon cycle, reefs, increasing CO2 in the air, killing fish, etc:
https://savethebaltic.wpcomstaging.com/2015/10/11/water-power-idustry-is-not-creating-green-electricity-it-creates-mordor/
Scroll down and read at least the yellow highlighted text in this:
https://www.maine.gov/dep/ftp/projects/necec/public-interest/2019-02-14%20%20From%20Steve%20Kasprzak%20KaAttachment%202%20%20Hydro-Quebec's%20Dams%20Chokehold%20On%20The%20Gulf%20of%20Maine.pdf
Lot more on Al Gore’s internet. ;)
#11, KIA–
Interesting, but there’s a whole lot of hand-waving going on there, plus some reasoning that looks a bit ‘motivated’ to me.
I will say that I particularly loved the bit from the second link that said:
Climate change sure can be a bitch, eh?
12 – Kevin McKinney
I found that information because I heard Dr. Joel Wallach describe why the world’s ocean reefs are dying. He was on the “Coast to Coast” radio show. You can hear his explanation in link below. Go to “Hour 2” audio, scroll over and listen from 12:15 to 16:02 (~4 minutes). Interesting theory:
https://criticalhealthnews.com/health-news/coast-to-coast-appearances-audio/476-audio-dr-joel-wallach-on-coast-to-coast-am-february-24th-2020
Kevin,
Wow!That was from mrkia’s link? Mrkia, did you read your link thoroughly for comprehension?
I’ve been watching the sea surface anomaly web site for a while and there’s been some low temperature anomaly off shore the PNW down to S. California and Baja, Mexico, https://www.ospo.noaa.gov/data/sst/anomaly/2020/anomnight.2.24.2020.gif .
The cooler surface waters off of S. Cal and Baja are probably due to winds and upwelling though it extends offshore a long ways but are the cooler surface waters off of Washington, Oregon, BC Canada due to winds as well as freshwater and snow melt? If anybody knows I’d appreciate it.
The reason I wonder is I’m in the Puget Sound area and we’ve had greater precipitation than any I’ve seen here and an early Spring. Spring started two weeks ago in Redmond, Wa which is about 250′ above sea level. There’s been some recent snow in the mountains but for the last decade or so the snow has been melting earlier than it should.
Thanks, Charles
#14, Al–
Yes, that was included among the stuff in the second link he posted; some of that passage even had the yellow highlighting that KIA particularly requested we read. The relation to the stuff about dams seemed–tangential, at best, and contradictory at worst. But there it was, much to my surprise.
Find our planet interesting and want to learn more about its history and scientific discoveries? Check out CuriosityStream where you can kind non-fictional documentaries on any and every topic now. Use code: From Head To Curve
https://curiositystream.com/FromHeadToCurve
15 – Charles
“I’ve been watching the sea surface anomaly web site for a while and there’s been some low temperature anomaly off shore the PNW down to S. California and Baja, Mexico,… ”
Don’t know why it’s cooler, but I was surprised to see sea ice down to Hokkaido Island, Japan on that map. Wikipedia in last paragraph of “climate” says it is common:
https://en.wikipedia.org/wiki/Hokkaido#Climate
Redmond is running close to normal temps now:
https://www.wunderground.com/forecast/us/wa/redmond
Snow quantities in the Cascades/Olympics vary wildly from one year to another. I think it’s normal. One year hardly any snow, next year record snow. Get out and enjoy it – lots of good places nearby – Snoqualmie Pass is about an hour from Redmond – good snow hikes/snowshoes/ski trips around there. Ditto Stevens Pass, MRNP, etc. Learn about avalanches before you go and watch the NW avalanche forecast. Take a class from the Seattle Mountaineers if you are not knowledgeable. They used to offer great day trips every week – probably still do. I think Sierra Club may offer trips also, just don’t listen to their politics. ;)
The ice machine is steady as she goes:
https://www.wunderground.com/forecast/ca/resolute?cm_ven=localwx_10day
14 – Al Bundy
“Mrkia, did you read your link thoroughly for comprehension?”
Not really – I was looking for information to back up the audio I mentioned in comment #13. Dude, I get paid by the comment, you know that! ;)
Mr. Know It All,
I’m not surprised by your comment. It reminded me of the Lake Pontchartrain: really wide but shallow.
For example, “I was surprised to see sea ice down to Hokkaido Island, Japan on that map.”
That’s not ice. NOAA says the white spaces on their anomaly maps indicate that “White and very light areas were near their three-decade average”.
And even the local Climate Change denier/fossil fuel apologist/weather scientist says the ten day map offered by wunderground is useless. I find the next day graphic on their maps useful for predicting when the next rain arrives but not much else.
Here are some links that you should read up on; https://www.climate.gov/maps-data/data-snapshots/data-source-monthly-sst-anomaly-global , https://climate.rutgers.edu/snowcover/chart_anom.php?ui_set=0&ui_region=nhland&ui_month=1. , https://www.ncdc.noaa.gov/teleconnections/pdo/ .
Wunderground is weather, not climate. But don’t worry, I did get a laugh from reading your comment and now I know that I can skip any of your future comments as a waste of time.
I’m a developmental biologist and my worry would be wether infant pteropods with very small, thin shells might find it harder to get going and grow. If Aragonite is so much more soluble than calcite we might see the proportion of pteropods decline and since that will reduce the ocean’s ability to buffer the CO2 could constitute a feedback tipping point. Once that process starts it will not stop unless and until we return the atmosphere to pre 1900 levels.
Even then as what happened to the cod on the Grand Banks shows things may not just go back to where and how they were. For those not in the know overfishing of cod on the Grand Banks resulted in a huge increase of biomass of salps, tubes of jelly. Salps eat baby cod but adult cod eat salps. We reduced the number of cod to the extent that the remaining cod are too few to breed and eat their way out of a small population. Short of fishing for salps (inedible) en mass and at enormous expense and effort there is no way out of this. We have ruined the Grand Banks as a food source for humans with our greed.
I could add in the rise of Humboldt squid off Southern California after so many sharks, swordfish and sailfish were removed as top predators. Humboldt are large, red, aggressive and their flesh is full of ammonia, so not good to eat. We did this.
#19 Charles Hollahan
Charles Hollahan:
‘That’s not ice. NOAA says the white spaces on their anomaly maps indicate that “White and very light areas were near their three-decade average”’.
Isn’t this about the map from the link posted by yourself at #15?
On the map is the text: “(white regions indicate sea-ice)”.
To compare I made a quick check at NSIDC Sea Ice Index:
https://nsidc.org/data/seaice_index/
…and at MASIE,
Multisensor Analyzed Sea Ice Extent – Northern Hemisphere (MASIE-NH):
https://nsidc.org/data/masie
All three maps show approximately the same amount of sea-ice that stretches all the way to the northern coast of Hokkaido. The map from NSIDC also shows median ice edge 1981-2010 and indicates that this year’s sea-ice extent in the Sea of Okhotsk is slightly less.
Muscleguy,
Yes, we did and are still doing it. For example, I eat a lot of skin-on wild salmon. I suspect that the ongoing changes in temperatures and currents are beyond the ocean’s ability to retain anything close to the mix of species we co-evolved with. Adding overfishing to the mix is foolish. An ocean full of hydrogen sulfide and stinging jellyfish MUST be avoided.
But I want my Omega-3.
19 – Charles Hollahan
“That’s not ice. NOAA says the white spaces on their anomaly maps indicate that “White and very light areas were near their three-decade average”.”
Charles, the link you provided in comment 15 goes to a map. Immediately below the map title at the top, in plain old Engrish is this phrase: “white regions indicate sea-ice”. Kind of unambiguous ain’t it?
You have been corrected by Mr. Know It All. Considering the rudeness of your comment, it is well deserved. ;)
On the Japanese sea ice: yes, that’s sea ice on the northern Japanese coast. But it’s not unusual at all.
Refer to the NSIDC map previously linked, here:
https://nsidc.org/data/seaice_index/
Northern Japan is at the top of the map. A moderately close look will reveal lines–NSIDC says magenta, but they look orange to me, at least this morning on this display. Those are the 30 year norms for ice extent, and you’ll see that they’re not all that far from the ice edge. There’s a bit more ice than usual in that region, but not hugely more.
More generally, it’s been a relatively good winter for sea ice extent, per VISHOP. We’ve had jet stream that’s behaved more decorously this year than in many recent years, and that’s meant that we haven’t seen the huge positive temperature excursions featured in some recent freeze seasons. The extent reflects that; as of yesterday (3/3/20), we were sitting at 14.45 million km2, well above the record low extent for the date of 13.82, recorded in 2016. We’re even below the average for the 2010s, I was surprised to note–that number is 14.07 km2.
We’d better enjoy it while we can, though, as a high (or low) extent now has limited predictive value for the minimum–2012 still holds the record for lowest extent so far, and on 3/3 2012 was sitting at 14.7 km2–above even the 2000s decadal average.
https://ads.nipr.ac.jp/vishop/#/extent/&time=2020-03-03%2000:00:00
Al Bundy,
But are your salmon wild caught or farmed? If farmed are they sea farmed or farmed on land with the effluent treated? Salmon farms are bad because of all the excess food and fish wastes they produce. Not to mention here in Scotland and Norway salmon fluke parasites endemic on farmed fish have spread to wild fish as the wild ones have to swim near the farms to get to their home rivers to breed.
Where I used to live in NZ, Dunedin introduced salmon smelt into the upper harbour (long and thin) using public subscriptions. When the salmon run you can catch them, no license required, in the harbour or at its narrow entrance. Though you may well be asked if you contributed to the last public subscription. Freeloaders are frowned on.
Salmon in NZ are introduced so are not vital to the environment. We no longer have bears in Scotland but we have otters, foxes, wildcats and others who all eat salmon if they can catch it. In the NA West Coast of course salmon running up the rivers move a huge amount of biomass and nutrients from the ocean into the forests as the bears, eagles etc eat them.
Also other oily fish contain omega 3’s, salmon are not the only ones. Which reminds me, I haven’t had a kipper or Arbroath smokie for a while. Both herring and haddock respectively are more sustainable than salmon, unless you catch it with a rod yourself.
Muscleguy,
I avoid farmed salmon like the plague.
I’ve gone salmon fishing. The captain was good (and/or got lucky) and we ended up in a very hungry school. No bait needed, just dip your hook in and jerk it up and down just so. I caught the limit quickly and then started giving my smallest fish to those who didn’t have the touch.
On the run home (I lived on Vancouver Island) a dolphin decided to ride our bow wave. I laid down and could have reached out and touched it but didn’t want to risk causing a crash at maximum speed. Twas amazing.
And yes with regard to way small oily fish, though I haven’t gone there yet. The rule of thumb is that the smaller the fish the less mercury and, as you noted, the more sustainable. Maybe I’ll make the switch, but inertia is sooo comfortable.
Lots of good vegetable oils, too. Cold-pressed olive, hemp, and flax come to mind.