The thing that really gets me in the gut about global warming from fossil fuel combustion is how long it will last. Carbon mined from the deep Earth and injected into the “fast carbon cycle” of the atmosphere, ocean, and land surface will continue to affect atmospheric CO2 concentrations, and climate, for hundreds of thousands of years into the future, unless we clean up the atmosphere ourselves.
It turns out that human emissions of the element mercury (Hg) will elevate mercury concentrations in the environment, and in upper trophic-level seafood, for thousands of years into the future. There are a lot of parallels to the carbon cycle. But, unlike the carbon cycle, the mercury cycle would be impossible to clean up.
The astonishing thing about the heavy metal, mercury, is how unheavy it seems to be as it runs around in the environment (Blum, 2013). Almost as massive as uranium, which they make artillery slugs and armor out of, mercury is a liquid at room temperature, and it can even evaporate into the air, plus dissolve in water. These tricks give it global mobility.
Mercury vapor, in the uncharged metal form written as Hg0, is chemically unstable in air, and it tends to oxidize (“rust”) to Hg2+, a charged ion that sticks to particles and dissolves in droplets, and rains out. But Hg2+ does an amazing trick called photo-reduction, absorbing a photon of ultraviolet sunlight and popping back up the energy hill to the Hg0 vapor phase1. Photo-reduction of Hg2+ allows mercury to float around in the atmosphere for about a year, enough time to deposit all around the world (Horowitz et al., 2017).
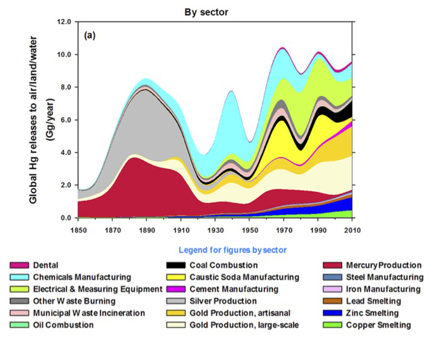
The global footprint of mercury deposition makes it harder to motivate ourselves to reduce emissions, in a tragedy of the commons that is totally analogous to the carbon cycle. Why (one may ask, and I will attempt to answer) should we clean up the mercury emissions from our coal plants when there are coal plants emitting mercury in China? And what’s up with “artisanal gold mining”?? (Image from Streets et al., 2017).
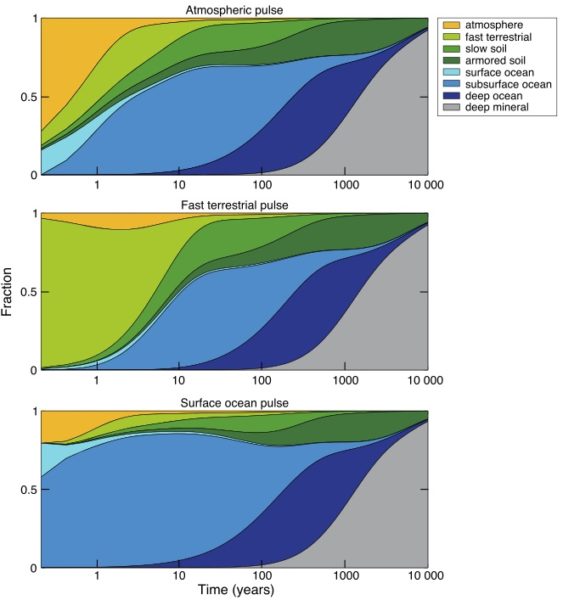
But once mercury does go to ground, it’s only the beginning of the story (Blum, 2013). Both the ocean and the land surface act like storage reservoirs in the mercury cycle, taking up mercury now when there’s a lot of it flying around, to release it back to the environment on various time scales in the future, some of them very long.
The ultimate removal pathway for mercury is deposition in ocean sediments, which is a pretty small flux relative to other fluxes in the mercury cycle. So, just like carbon, the “fast” surface cycle gets charged up with the extra load (mercury or carbon), until the slow leak flux to the solid Earth, by way of ocean sediments, finally cleans up the load. For mercury, the clean-up time scale is probably about 10,000 years (Amos et al., 2013).
Because of mercury’s tendency to recycle after it deposits, today there is more mercury deposition called “legacy anthropogenic”, meaning recycled from emission decades ago, than there is deposition of mercury we are emitting now. So just like for carbon, we are creating an accumulating load in the mercury cycle.
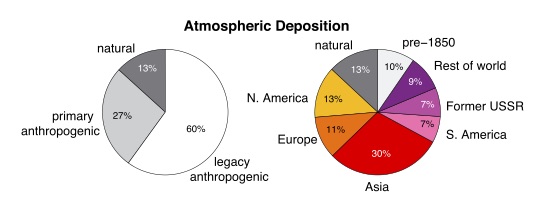
From (Amos et al., 2013), showing the origins of global mercury deposition, “when from” on the left, and “where from” on the right.
Mercury deposition on land is primarily through mercury vapor uptake by plant leaves (called “dry deposition”, (Demers et al., 2007)). The mercury is carried to the ground in leaf litter, and it collects in the soil organic carbon pool. Soil organic carbon cycling is important in the carbon cycle as well, so it has been well studied, but it is a complicated world. The fate and lifetime of organic carbon in a soil is very different in, for example, a depositional swamp versus a rain-scoured and eroding hillside.
On a global scale, what happened to bomb radiocarbon (14C) from nuclear weapons testing in the 1960’s can tell us a lot about the behavior of the soil organic carbon system. It can be described in broad brush by a simple reservoir or “box” model, consisting of several reservoirs with differing carbon production rates, and turnover times ranging from years to thousands of years, with names like “slow”, “fast”, and “armoured” (Smith-Downey et al., 2010). The mercury attached to the carbon is re-released to the environment, primarily as dissolved Hg2+, when the organic carbon degrades. The Hg2+ will probably be carried to the ocean and eventually recycled from there. Mercury that goes to ground in the longest-lived organic carbon pools will continue to dribble back out to the environment for thousands of years.
In the ocean, most of the mercury that falls to the ocean surface gets quickly returned to the atmosphere, by photo-reduction of Hg2+ in the surface ocean, producing Hg0 that “evades” (think evaporates) (Soerensen et al., 2010). The elevated mercury deposition rates today have driven up the concentration of Hg2+ in the surface ocean, and the load has been carried subsurface by ocean flow. The mercury load near the sea surface also sticks to sinking particles, re-dissolving at the depths where the organic carbon in the particles degrades. When high-mercury subsurface water eventually comes back to the surface, it releases its mercury back to the atmosphere (Cossa et al., 2004). The ocean therefore acts as another mercury reservoir, which will recycle human-mined mercury back to the atmosphere for thousands of years.
Surprisingly, given that we live on land, most human exposure to mercury comes from the ocean mercury cycle, by way of seafood. Mercury bio-accumulates in fish in the form of mono-methyl mercury (MMHg, chemical form CH3Hg+). This is a quantitatively relatively minor species with an outsized impact, a little bit like methane, maybe? OK, that’s weak. MMHg is produced by bacteria, and degrades quickly enough that MMHg and Hg2+ coexist in a quasi-equilibrium in the ocean, with about 5-10% of mercury in the methylated form in most places and depths (Semeniuk and Dastoor, 2017; Archer and Blum, 2018). MMHg is the form taken up by phytoplankton and amplified up the food chain, to the point that higher trophic-level fish like most tuna, orange roughy, sea bass, swordfish, and shark are toxic to eat too often.
When we realize that we have degraded the climate system by releasing fossil carbon, we can theoretically clean it up. Although there may not be enough agricultural land to do it entirely with the currently fashionable option, bio-energy carbon capture and sequestration (BECCS), there is always chemical removal of CO2 from the atmosphere (Keith et al., 2018). Cleaning up the anthropogenic carbon is theoretically feasible because a relatively large fraction of the anthropogenic carbon, a little more than half, is still in the atmosphere. If we pulled enough CO2 out of the atmosphere, the oceans would degas CO2, slowing down the atmospheric recovery. But it would be theoretically feasible, carbon-cycle wise, to pull atmospheric pCO2 down to the 350 ppm “safe” level in a few decades2.
The one-year atmospheric lifetime of mercury is much shorter than the drawdown lifetime for carbon (decades to centuries: (Archer et al., 2009)). For this reason, a much smaller fraction of all of the anthropogenic mercury is still in the atmosphere, only about 1.5% (from Amos et al., 2013). Hg0 vapor is out of equilibrium and photo-sensitive, so it would presumably be feasible to scrub mercury from air alongside CO2 in the chemical atmosphere-scrubber plants. But most of the anthropogenic mercury has already gone to ground, on land and in the oceans, and until it dribs and drabs back into the environmental mercury cycle over thousands of years, it will be out of our reach, making a quick cleanup impossible.
And since the atmospheric Hg0 concentration is controlled by the mercury cycle with a time constant of just one year, we could never move the needle of the atmospheric Hg0 concentration, or therefore mercury deposition rates or seafood mercury loads. Once the Hg is released into the biosphere, it can only be endured, for thousands of years3. My personal feeling is that if there were any grown-ups on the planet, we wouldn’t be allowed to do this.
1I was moved to compose a haiku about Hg2+ photo-reduction.
Mercury, aloft
Tagged by bromine radical
Zap! Ha! I am gone!
The bromine radical chemistry referred to in the poem occurs mostly in Arctic springtime (Horowitz et al., 2017), a phenomenon analogous to ozone hole chemistry with chlorine.
2The on-line interactive ISAM carbon cycle / integrated assessment model (Cao and Jain, 2005) predicts that about 440 Gton CO2 would have to be removed from the atmosphere to achieve a concentration of 350 ppm within 20 years.
3I was moved to compose a limerick about the global mercury cycle
The mercury cycle goes ‘round it
A global load, and we pound it
I’ve come to believe
Our plan is to leave
A world crazier than we found it
The craziness referred to in the poem refers to neurological impacts of mercury exposure such as “mad hatter’s disease”.
Amos, H. M., Jacob, D. J., Streets, D. G., and Sunderland, E. M.: Legacy impacts of all-time anthropogenic emissions on the global mercury cycle, Global Biogeochemical Cycles, 27, 410-421, 10.1002/gbc.20040, 2013.
Archer, D., and Blum, J.: A model of of mercury cycling and isotopic fractionation in the ocean, Biogeosciences, 15, 6297-6313, 10.5194/bg-15-6297-2018, 2018.
Archer, D. E., Eby, M., Brovkin, V., Ridgewell, A. J., Cao, L., Mikolajewicz, U., Caldeira, K., Matsueda, H., Munhoven, G., Montenegro, A., and Tokos, K.: Atmospheric lifetime of fossil fuel carbon dioxide, Ann. Reviews Earth Planet Sci., 37, 117-134, 2009.
Blum, J. D.: Mesmerized by mercury, Nature Chemistry, 5, 1066-1066, 10.1038/nchem.1803, 2013.
Cao, L., and Jain, A.: An Earth system model of intermediate complexity: Simulation of the role of ocean mixing parameterizations and climate change in estimated uptake for natural and bomb radiocarbon and anthropogenic CO2, Journal of Geophysical Research-Oceans, 110, 10.1029/2005jc002919, 2005.
Cossa, D, Cotte-Krief, MH, Mason, RP, Bretaudeau-Sanjuan, J.: Total mercury in the water column near the shelf edge of the European continental margin, Marine Chemistry 90: 1-4, pages 21-29,DOI: 10.1016/j.marchem.2004.02.019, 2004
Demers, JD, Driscoll, CT, Fahey, TJ, Yavitt, JB.: Mercury cycling in litter and soil in different forest types in the Adirondack region, New York, USA, Ecological Applications 17: 5, pages 1341-1351, DOI: 10.1890/06-1697.1, 2007
Horowitz, H. M., Jacob, D. J., Zhang, Y. X., Dibble, T. S., Slemr, F., Amos, H. M., Schmidt, J. A., Corbitt, E. S., Marais, E. A., and Sunderland, E. M.: A new mechanism for atmospheric mercury redox chemistry: implications for the global mercury budget, Atmospheric Chemistry and Physics, 17, 6353-6371, 10.5194/acp-17-6353-2017, 2017.
Keith, D., Holmes, G., St. Angelo, D and Heidel, K.: A Process for Capturing CO2 from the Atmosphere, Joule (2018), https://doi.org/10.1016/ j.joule.2018.05.006, 2018.
Semeniuk, K., and Dastoor, A.: Development of a global ocean mercury model with a methylation cycle: Outstanding issues, Global Biogeochemical Cycles, 31, 400-433, 10.1002/2016gb005452, 2017.
Smith-Downey, NV, Sunderland, EM, and Jacob, DJ: Anthropogenic impacts on global storage and emissions of mercury from terrestrial soils: Insights from a new global model, Journal of Geophysical Research-Biogeosciences 115, Article Number: G03008, DOI: 10.1029/2009JG001124, 2010.
Soerensen, A. L., Sunderland, E. M., Holmes, C. D., Jacob, D. J., Yantosca, R. M., Skov, H., Christensen, J. H., Strode, S. A., and Mason, R. P.: An Improved Global Model for Air-Sea Exchange of Mercury: High Concentrations over the North Atlantic, Environmental Science & Technology, 44, 8574-8580, 10.1021/es102032g, 2010.
Streets, D. G., Horowitz, H. M., Jacob, D., Lu, Z. F., Levin, L., ter Schure, A. F. H., and Sunderland, E. M.: Total Mercury Released to the Environment by Human Activities, Environmental Science & Technology, 51, 5969-5977, 10.1021/acs.est.7b00451, 2017.
I am impressed that anthropogenic mercury now makes up greater than 85% of the mercury cycle. I am also impressed by the number of anthropogenic sources.
It would be good to see another article on lead, to illustrate the geochemical differences.
‘Cause the future wasn’t depressing enough if we don’t act quickly enough… and nobody wants to simplify… so… thanks.
:-(
“Almost as massive as uranium” : at room temperature, the density of mercury is 13.6
while density of uranium is 19.1
So, “almost” is probably not the most adequate.
[Response: I was thinking molecular weight, Hg is 80% of U. Whatever…David]
Lead just sort of sits there in the soils, doesn’t go anywhere. It is mostly toxic to growing brains, and not as toxic to adults, unlike mercury. All the great old guys in history, like Ben Franklin, were lead-poisoned by drinking wine distilled in lead, to sweeten the port with lead acetate “sugars of lead”. It gave them gout by interfering with an enzyme that secretes nitrogen, resulting in the buildup of ureate (uric acid) crystals in the joints. There is tricky galvanic chemistry that goes on in solder joints of plumbing, where dissimilar metals come into contact with each other directly and through the water. The issue in Flint, MI was that the SO42- / Cl– ratio in Lake Huron water (the original source of Flint drinking water) tended to prevent dissolution of lead, but when they switched to Flint River water, that ratio was more permissive of lead dissolution. It sounds like tricky chemistry, but it’s well-known in water treatment to watch for this, and they were supposed to add phosphate (PO43- to inhibit lead dissolution, and to monitor tap water from places with lead pipes, neither of which they did.
This is the most dubious thing I ever red from Real Climate.
Mercury is poisoneous, but it is not an element that remains and accumulates in the human body forever. Cadmium is much worse and can only be excreted over time by swetting. Thus if you swet a lot, it may be a symptom of chronical cadmium poisoning.
On spills of Mercury, open all doors and Windows, pick it up With a flattened brass or copper wire With tin- solder. It alloys and adsorbs eagerly to tin. This Message is not to be amputated here, because it is the old known Scientific way. Pick it up with a special “Mercury-picker” of proper tin.
Then use a strong vacuum cleaner With enough sulphurous sanitary bark or sawdust in the bag. Still With open doors and Windows,
Then go after it with Sulphur.
Polysulphide can be made by fusing elementary sulphur With caurtic and some water , and dissolved furher in water. Alkali sulphides can also quickly be made in case of accident by fusing sawdust and sulphate, eluate it with water.
Spray it everywhere , where you suspect Mercury.
HgS is stable in nature, else the spanyards would not have survived or maybe they had become mad hatters all of them, and not just a few of them.
There are landscapes full of Mercury.
The rather stable form is Zinnober, Mercury sulphide.
On working With Mercury, allways have it doubble secured. Never work With Mercury outside of a large proper and clean van of polyetylene, and With that tin- stick picking method at hand, grasped and trained.
We found a treatize from 1450 on portative organs in LATIN by Arnaut van Zwolle.
For the organ pipe metal solder, take 1 Ld of Plumbum and 2 Ld of Stannum he wrote, and fuse it. On solidifying you will see a figure on the top called “EVTECTICVM”. Then pour a QVANTVM MERCVRIVM into that EVTECTICVM and you have it.
But do it outdoor With Wind in Your back because “Vaporum est TOXIC”, he also wrote, in sheere mideival LATIN.
It is as easy as that.
In the lab, remenber that large and clean polyetylene van, the picking tin- stick, and the polysulphide method and spray.
Of course it settles in anaerobic sulpide muds then, and will be set free again when those are industrially burnt. But sulphide is what better binds it.
David might further explain how the remobolization and recycling effects he discusses apply to the volcanic and biogeochemical cycles of Hg.
To what degree is the volatilization and atmospheric entrainment of mercury from hot springs and fumaroles in proximity to large populations, like those in California and Tuscany, sensitive to rising ambient temperatures?
[Response: I’m not sure what the temperature impacts on the “fast mercury cycle” would be. Another possible climate connection that could turn out to be important is ocean temperature or oxygen levels or pH or whatever affecting the abundance of methyl mercury, and hence human exposure to mercury. David]
I know this is a climate science site that works hard to keep the topics relevant. But this is just the kind of article I’m also glad to see written here. The article points at an analogy to an aspect of climate science, of course. But I enjoyed learning some technical details about my own muddled understanding of mercury and our environment. This article informed me. And I appreciate it a lot.
This kind of injection, from time to time, is just the ticket for keeping this site from becoming wearying (not the fault of the authors, but the fault of the same people making similar arguments.) Keeping it infrequent, but periodic, adds a continuing science education element without losing sight of the mission and keeps it worth coming back, more often. And I’d personally appreciate that added difference.
— — —
On this note, a personal story.
Mount St. Helens is about 40 air-miles from where I’ve lived all my life, the Portland area. It was quite the drama here when it exploded one day in 1980. Many years later, I happened to be reading a science report on a different topic, geology, and encountered a chart displaying a number of different curves covering concentrations of various minerals and elements in several borehole records in Colorado and some nearby states. For the ones showing mercury concentrations vs historical time, it was interesting to me to see hand-written notes where Mount St. Helens was noted as the event explaining those peaks. Quite a spike was present there and I gathered from this that mercury was a significant element in the emissions from Mount St. Helens. I wasn’t shocked, just took note of an interesting detail about that event.
Perhaps these are the kinds of events that explain the “13% natural” part of the left pie chart.
Separately, Oregon is notable for another aspect regarding mercury. The 2nd largest mercury mine in the US was at Black Butte in southern Oregon (a federal super-fund site now), shut down in the 1960’s, I think, after operating for a century or so. The mercury pollution resulting from those mining activities have left Dorena Lake (it’s quite a ways away from black butte area) so polluted with mercury that to this day even a single fish from that lake is considered unsafe to eat. (I was actually considering buying the 800 acre piece that includes that mountain, years ago — before learning more details.)
Thanks david for the info on lead.
Also notable — improving disinfection caused problems.
https://www.washingtonpost.com/local/dcs-decade-old-problem-of-lead-in-water-gets-new-attention-during-flint-crisis/2016/03/17/79f8d476-ec64-11e5-b0fd-073d5930a7b7_story.html
Don’t be so sure lead has little effect on adults. The CDC is supporting the idea that no level of lead in humans is acceptable.
Clair Patterson was the first to develop techniques to accurately measure tiny concentrations of lead as part of his quest to determine the age of Earth.
When he discovered that preindustrial humans had far less lead in their bodies than all modern humans, he wrote: “It seems probable that persons polluted with amounts of lead that are at least 400 times higher than natural levels, and are nearly one-third to one-half that required to induce dysfunction, that their lives are being adversely affected by loss of mental acuity and irrationality. This would apply to most people in the United States”.
Plenty of other toxins in coal as well: lead, cadmuim and arsenic. Arsenic in particular is toxic in very, very small quantities. The Victorians of the 19th century used it in the green colour pigments in paint and wall paper, but it was phased out fairly quickly.
[Response: Arsenic can “evaporate” from that wallpaper in the form of AsH3, arsine gas. Might have killed Napoleon on Elba Island. David]
Informative, yet depressing.
It seems that whatever the future of life on this planet, it’s going to (have to) become more tolerant of mercury over the coming millennia.
[Response: I don’t know, the toxicity seems at a pretty fundamental level, biologically. We can’t just get rid of sulfur-containing amino acids. ]
Which leads to the question of impacts: we know that sufficiently high doses of mercury are extremely neuro-toxic to humans (‘Minamata disease’, or the Grassy Narrows tragedy in Canada, etc.) It’s easy to presume that this would extend to other mammals, and to fish and birds. What about arthropods? Invertebrates?
What do we know about the effects across various taxa?
[Response: I read a paper about fish being less able to reproduce when exposed to Hg (Depew et al, Environmental Toxicology and Chemistry, Vol. 31, No. 7, pp. 1536–1547, 2012), but fish in the ocean are so clobbered by fishing and warming and acidification and runoff and plastics (?) and everything else, it’s hard to isolate a single problem. David]
In talking with electrical power scientists (of the monopoly kind) several years back when we were promoting renenewable energy for our state’s portfolio they mentioned a trackable HG flume from China that was distinct(and excessively larger) than the US ambient level also with its own signature.
“A world crazier than we found it.” Thanks for the superbly informative post, and the limerick. The crazy behaviors that normalize human sources of mercury pollution lead to a physical world that will only multiply degenerative pathologies of bodies and minds.
Therefore, the crazy behaviors (words, acts, and Acts) that normalize human sources of mercury pollution become a self-fulfilling prophecy, as I’ve often thought. It’s a self-harming normality and a self-normalizing harm. It’s, well, maddening.
Ditto: lead.
I’m thinking that if we did create a global Mercury removal program, the small airborne fraction and short airborne residential time might not be a game stopper. We would just have to continue the removal program for a very long time (thousand years or more), but we could in shirt order reduce the airborne concentration quite significantly and keep it there as long as we keep the program active. To the extent that human exposure is governed by atmospheric load this would be both beneficial and easy to sell.
[Response: I’m thinking that since the Hg cycle replenishes the atmosphere in a year, we’d have to remove mercury from the entire volume of the atmosphere in a year or less if we were to pull Hg down below what the Hg cycle “wants”, which would be impossible. I think it would be like spitting into a hurricane. David]
https://www.cnn.com/2018/06/13/health/falling-iq-scores-study-intl/index.html
I recall a Clifford Simak novel in which friendly aliens offered to induce stupidity in the human species, as a kindness.
Great time pulse infographics. Thank you.
While mercury is bad at typical environmental levels, we might also want to pay more attention of the metal lead, especially in users of firearms, because their ingestion of lead, and the subsequent effect of their lead influenced behavior on society, is a serious unreported problem. Shooters absorb far more lead than the rest of us, from a combination of breathing lead fumes from primer, breathing lead fumes that lead projectiles leave as they exit the barrel, and skin absorption from routine cleaning of weapons. There are plenty of studies showing elevated blood lead levels in shooters, and lead attacks brain synapses. Just knowing these two things, it should be clear that we have a largely hidden or under reported threat to the neurological health of a large portion of our nation. Think, for instance, of the high suicide rate among soldiers. Think too of the fact that most if not all mass shootings are committed by people who are very likely to have elevated blood lead levels. Mercury is definitely a major threat, but in the short term, I am even more concerned about the concentration of Pb in the brains of so many people. The potential for diminished intellectual capacity in such a large group demands our concern.
http://www.atimes.com/article/coal-dust-is-tainting-chinas-rice-with-mercury/
17 – SteveP
If you’re worried about lead poisoning, don’t worry about firearms – worry about poisoning from occupational exposure:
https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6247a6.htm
Firearms aren’t a problem. With 600 million firearms and 24 trillion rounds of ammo owned by US citizens, if firearms were a problem, you’d KNOW it! ;)
https://bearingarms.com/jenn-j/2017/06/27/more-than-600-million-firearms-in-america/
Indoor shooting ranges are becoming more aware of the need for better ventilation, etc to keep lead levels down. And non-lead ammo is available including with non-lead primers:
http://huntingwithnonlead.org/
https://www.livescience.com/42117-green-bullets-hunting-non-lead-ammo.html
I think I’ve read in a few places that animals deal with mercury by sequestering it in their livers reacted with selenium, so that selenium becomes important for organisms to have enough of, in mercury-contaminated environments.
Has anyone else come across such?
Brian
MR KIA @19 says firearms aren’t a problem, followed by indoor shooting ranges are becoming more aware of the need for better ventilation, etc to keep lead levels down.
Sigh. If firearms aren’t a problem, why would you need better ventillation etc?
In fact multiple scientific studies raise concerns that lead in bullets is a problem for human health:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5161761/
@ Brian Cady 9 Nov 2018 at 1:40 PM
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3654245/
“(v) Selenium is an important essential element, that is present at a broad range of levels across populations. The selenide ion forms an extremely stable, insoluble compound with mercury, and provides relief of mercurialism symptoms. On the face of it, selenide might not be compatible with chelation, as the two agents may counter the effectiveness of one another [62]; however, selenium may be incorporated in organic molecules, and organic selenium/mercury complexes may be transported through membranes. Selenium depletion in the face of mercury exposures also depletes seleno-enzymes. In humans, organic selenium supplementation was beneficial in a controlled trial among 103 mercury-exposed villagers [63]. A selenium yeast product increased mercury excretion and decreased oxidative stress-related biomarkers urinary malondialdehyde and 8-hydroxy-2-deoxyguanosine [63].”
I wonder what the cost/benefit to live-catch high mercury seafood, then apply chelation to eliminate mercury before processing them as people food.
I envision a wild caught farm finished & detoxified process chain which would also remove some mercury from the environment.
#19, KIA–
So many punchlines, so little time…
For Brian Cady, LMGTFY:
https://www.google.com/search?client=firefox-b-1-ab&q=animals+deal+with+mercury+by+sequestering+it+in+their+livers+reacted+with+selenium
This is peripheral, but mentions selenium’s role in protecting against mercury toxicity.
https://www.sciencedirect.com/science/article/abs/pii/S0165993617302364
Three more on this:
https://doi.org/10.1139/A08-001
https://doi.org/10.1016/j.cbi.2015.02.001
https://pubs.acs.org/doi/abs/10.1021/es200478b
Brian
But as selenium is toxic in and of itself, it’s scarcely the best way to approach limiting mercury poisoning.
Be wary of the Internet “doctors” out there pushing selenium as a dietary supplement; they’ve made some people very sick.
23
Kevin McKinney says:
10 Nov 2018 at 10:48 AM
#19, KIA–
Firearms aren’t a problem. With 600 million firearms and 24 trillion rounds of ammo owned by US citizens, if firearms were a problem, you’d KNOW it!
So many punchlines, so little time…
———-
I wanna see all those firearm owners form a really big circle inside the US, place KIA right in the middle of that circle, and let loose with those 24 trillion rounds all at once!
There’s like several thousands to millions of problems the US has solved in one go. :-)
Here’s the thing few have noticed: all the carbon we have been putting into the atmosphere was removed from the atmosphere over millions of years. Carbon was put into the atmosphere 250 million years ago from huge volcanic flows that lasted perhaps for a million years, but definitely covered an area equal to the continental United States, thousands of feet deep (the Siberian Traps). It caused one of the great mass extinction events. The carbon was scrubbed from the atmosphere and sequestered as coal, oil, and natural gas. Now we have put a lot of that carbon back into the atmosphere where it can cause another mass extinction event. Congrats!
Mercury doesn’t kill people. People kill people. So to speak.
Isn’t it something how, with gun fatalities and injuries, the spin blames all the harm purely on human agency (“guns don’t kill people, people kill people”) but with climate disruption and many kinds of pollution, the spin will fault anything and everything except human agency?
The dichotomy is false. (A person plus a gun–or a gun plus a person–is what kills people.) In any case I think that this kind of ubiquitous dichotomy is the linguistic trace of a divide-and-conquer strategy that lurks behind the spin in the first place–consciously or not.
Non-lead ammunition is token. The push for lead (“traditional”) ammunition remains.
https://undark.org/article/lead-ammunition-bullets-hunting-copper/
http://projects.seattletimes.com/2014/loaded-with-lead/1/
Lead serves no purpose at all in the human body, in any amount. It is functionally a pure toxin, a pure poison.