Some of you might have read about the lawsuit by a number of municipalities (including San Francisco and Oakland) against the major oil companies for damages (related primarily to sea level rise) caused by anthropogenic climate change. The legal details on standing, jurisdiction, etc. are all very interesting (follow @ColumbiaClimate for those details), but somewhat uniquely, the judge (William Alsup) has asked for a tutorial on climate science (2 hours of evidence from the plaintiffs and the defendents). Furthermore, he has posted a list of eight questions that he’d like the teams to answer.
It’s an interesting list. They are quite straightforward (with one or two oddities), but really, pretty much textbook stuff. Andrew Dessler made a quick stab at answering them on Twitter:
Here are answers to questions posed by the Judge Alsup re: climate science (https://t.co/DLFDT70PdL). Turns out answers to those questions are actually pretty well known. 1/
— Andrew Dessler (@AndrewDessler) March 8, 2018
But I think we can do better. So what I propose is that we crowd-source the responses. They should be pithy, to the point, with references (not Wikipedia) and, preferentially, accompanied by a good graphic or two. If we can give a credible uncertainty to any numbers in the answer that’s a bonus. I’ve made a start on each, but further voices are needed. Put your response in the comments and I’ll elevate the best ones (giving credit of course) to the main post. If you have any other comments or edits to suggest, feel free to do so. The best of those will also be incorporated. [Update: I realise I can’t possibly incorporate all the good suggestions while still keeping this short. So be sure to read the comments too for additional material. Also, as I should have said to start with, the best responses to these kinds of questions (though not to these specifically) are to be found in the FAQ of the IPCC report, the Royal Society/National Academies report, and the US. National Climate Assessment science report.]
Alsup’s Questions:
- What caused the various ice ages (including the “little ice age” and prolonged cool periods) and what caused the ice to melt? When they melted, by how much did sea level rise?
- What is the molecular difference by which CO2 absorbs infrared radiation but oxygen and nitrogen do not?
- What is the mechanism by which infrared radiation trapped by CO2 in the atmosphere is turned into heat and finds its way back to sea level?
- Does CO2 in the atmosphere reflect any sunlight back into space such that the reflected sunlight never penetrates the atmosphere in the first place?
- Apart from CO2, what happens to the collective heat from tail pipe exhausts, engine radiators, and all other heat from combustion of fossil fuels? How, if at all, does this collective heat contribute to warming of the atmosphere?
- In grade school, many of us were taught that humans exhale CO2 but plants absorb CO2 and return oxygen to the air (keeping the carbon for fiber). Is this still valid? If so, why hasn’t plant life turned the higher levels of CO2 back into oxygen? Given the increase in human population on Earth (four billion), is human respiration a contributing factor to the buildup of CO2?
- What are the main sources of CO2 that account for the incremental buildup of CO2 in the atmosphere?
- What are the main sources of heat that account for the incremental rise in temperature on Earth?
Alsup’s Answers:
Note this is an updating text. Last edit: March 16, 2018
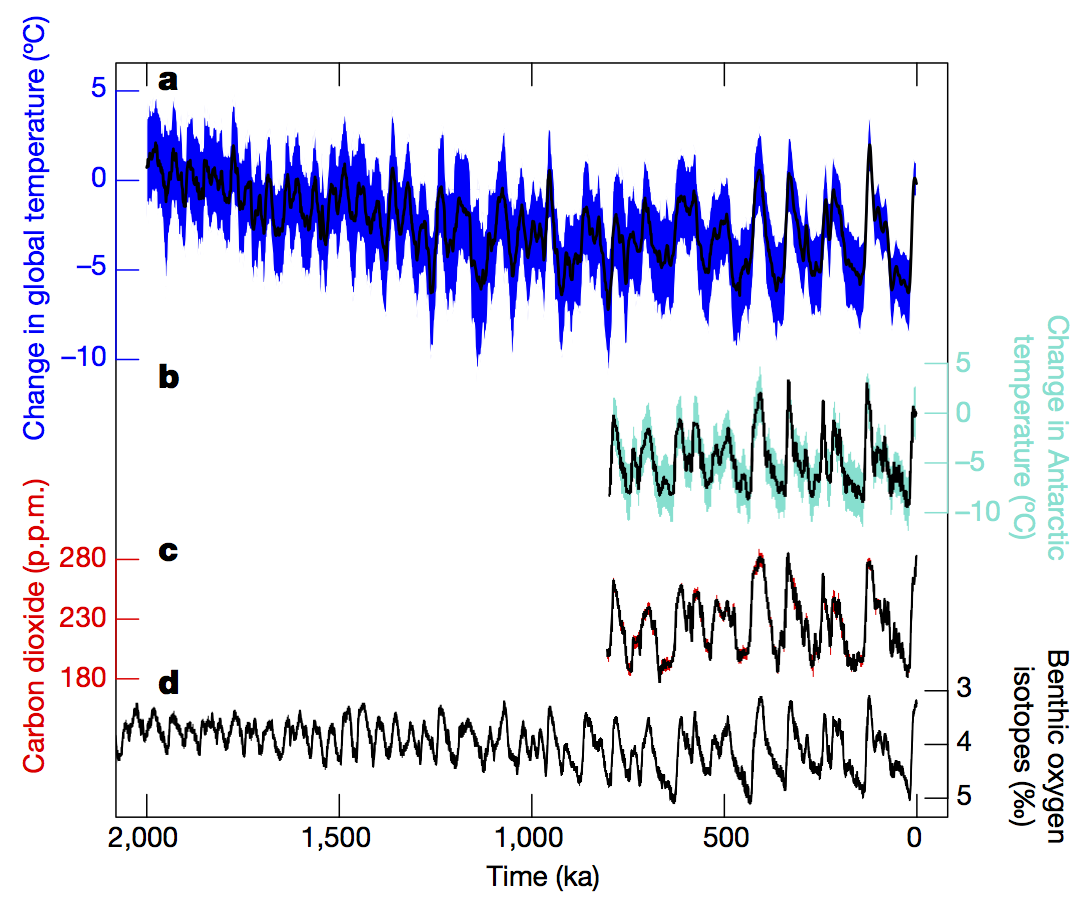
- The “ice ages” are the dominant cycles of change over the last 2.5 million years (Snyder, 2016):

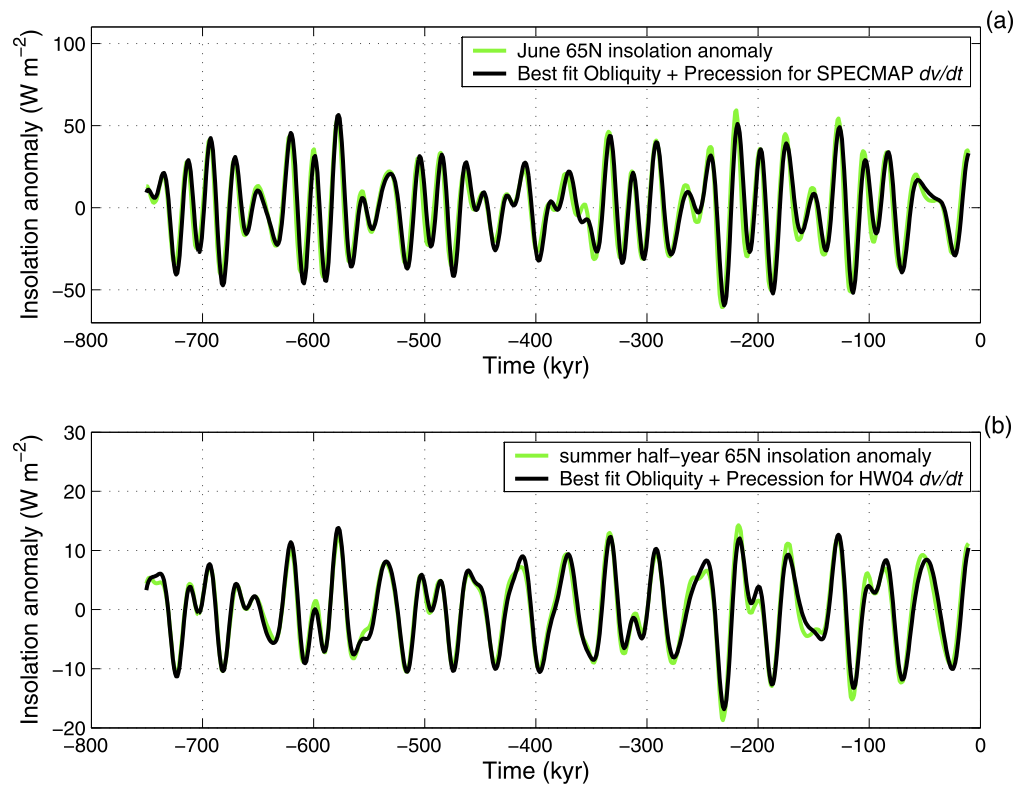
They vary in extent and duration. They generally were larger in the last 800,000 years, and the duration changed from about 40,000 years in the first half to about 100,000 years in the later period. It was discovered in the 1970s that the cycles were highly correlated to changes in the variability of the Earth’s orbit – the so-called Milankovich cycles (Hays, Imbrie and Shackleton, 1976). More recent work has shown that the growth and collapse of the ice sheets is strongly tied to the incoming solar radiation (insolation) at high latitudes (Roe, 2006):

The magnitude of the cycles is strongly modified by various feedbacks, including ice-albedo, dust, vegetation and, of course, the carbon cycle which amplify the direct effects of the orbital changes. Estimates of the drivers of global temperature change in the ice ages show that the changes in greenhouse gases (CO2, methane and nitrous oxide) made up about a third of the effect, amplifying the ice sheet changes by about 50% (Köhler et al, 2010).The sea level changes over these cycles was large. The difference between the last glacial maximum (20,000 yrs ago) and today is about 120 meters (400 ft), but the high levels during some of the warmest interglacials were 6-9 meters (20 to 30 feet) higher than today. These changes are dominated by the amount of ice volume change.
The so-called “Little Ice Age” was a cooling of the Northern Hemisphere climate (and possibly less markedly in the Southern Hemisphere) in the period of the fourteenth century to the the 1850’s, approximately. It came after a period of a relatively warm climate called the Medieval Warm Period. The cause of this relatively short lived cooling (it was not a true “ice age”) is likely due to an increase in volcanic eruptions and with some role for a slightly reduced solar activity. Over the Holocene (last 11,000 yrs) there is a small but persistent cooling trend due to the orbital cycles mentioned above.
- Greenhouse gases are those that are able to absorb and emit radiation in the infrared, but this is highly dependent on the gases molecular structure. Vibrational modes in molecules with three or more atoms (H2O, CO2, O3, N2O, CH4, CFCs, HFCs…) include bending motions that are easier to excite and so will absorb and emit low energy photons which coincide with the infrared radiation that the Earth emits. Thus it is these molecules that intercept the radiation that the Earth emits, delaying its escape to space. More detailed discussion including the importance of the gases dipole moment can be found here. Diatomic molecules (like N2 or O2) have stretching modes (with the distance between the two molecules expanding and contracting), but these require a lot of energy (so they absorb only at higher energies. Some absorption is possible in the infrared due to collisions but calculations suggest this is a very small part (~0.2%) of the overall greenhouse effect (around 0.3 W/m2, compared to a total effect of 155 W/m2) (Höpfner et al, 2012).

Figure showing the vibrational modes for CO2. Arrows indicate the directions of motion. Vibrations labeled A and B represent the stretching of the chemical bonds, one in a symmetric (A) fashion, in which both C=O bonds lengthen and contract together (in-phase), and the other in an asymmetric (B) fashion, in which one bond shortens while the other lengthens. The asymmetric stretch (B) is infrared active (allowed by quantum mechanics) because there is a change in the molecular dipole moment during this vibration. Infrared radiation at 2349 (4.26 um) excites this particular vibration. The symmetric stretch is not infrared active, and so this vibration is not observed in the infrared spectrum of CO2. The two equal-energy bending vibrations in CO2 (C and D) are identical except that one bending mode is in the plane of the paper, and one is out of the plane. Infrared radiation at 667 (15.00 um) excites these vibrations. (source)
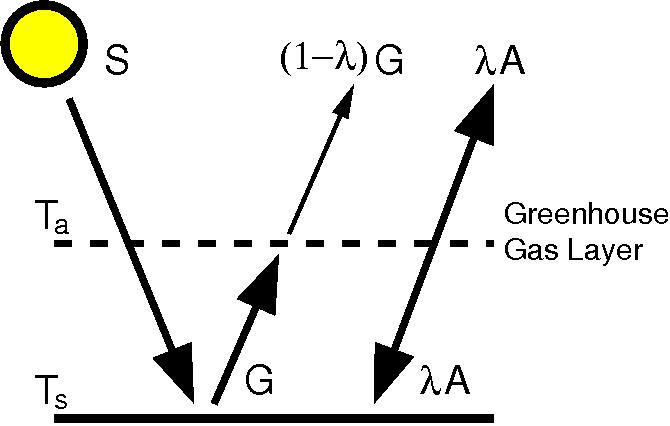
- The Earth’s surface emits infrared radiation. This is absorbed by greenhouse gases, which through collisions with other molecules cause the atmosphere to heat up. Emission from greenhouse gases (in all directions, including downwards) adds to the warming at the surface.

The figure shows the easiest mathematical description of the greenhouse effect. The downward radiation from greenhouse gases can be easily measured at the surface in nights under clear skies and no other heat sources in the atmosphere (e.g. Philipona and Dürr, 2004).
- Yes, but not enough to matter. The latest update to the estimates of radiative forcing of CO2 (Etminan et al., 2016) shows a shortwave effect (i.e. a change in the absorption of downward solar radiation) is about -0.14 W/m2 for CO2 going from 389 to 700 ppm (compared to 3.43W/m2 in longwave forcing) – contributing to about a 4% decrease in the net forcing.
- Direct heat generated by the total use of fossil fuels and other forms of energy adds up to about 18TW [IEA,2017]. Spread over the planet that is 0.04W/m2. Compared to anthropogenic forcings since 1750 of about 2.29±1.1W/m2 [IPCC AR5, Figure SPM 5], it’s about 1/100th the size. Locally however (say in cities or urban environments), this can be more concentrated and have a bigger impact.
- The grade school calculation is still valid. All animals (including humans) breathe in oxygen and exhale CO2. The carbon in the exhaled CO2 comes from the food that the animals have eaten, which comes (ultimately) from carbon that plants have taken from the atmosphere during photosynthesis. So respiration is basically carbon neutral (it releases CO2 to the atmosphere that came from the atmosphere very recently). Plants do take up CO2 as they are growing. With higher CO2 concentrations (and higher temperature), plants in fact increase their CO2 uptake somewhat but not as much as would be needed to absorb all the human-caused emissions. Of these emissions only about a quarter is absorbed by plants, while another 20% is absorbed by the oceans, but about half of the emissions stay in the atmosphere.
Note that any net change in biomass (whether trees, or cows or even humans) does affect atmospheric CO2, but the direct impact of human population growth is tiny even though our indirect effects have been huge. For scale, the increase of 3 billion people over the last 40 years, is equivalent to:
0.185 (fraction of carbon by mass) * 80 kg (average mass of a human) * 3 billion (additional humans) * 10-3 (conversion to GtC) / 40 years = 0.001 GtC/yr
which, compared to current fossil fuel and deforestation emissions of ~10 GtC/yr is 4 orders of magnitude too small to be relevant.
- Main sources of human CO2 emissions are fossil fuel burning and (net) deforestation. This figure is from the Global Carbon Project in 2017.

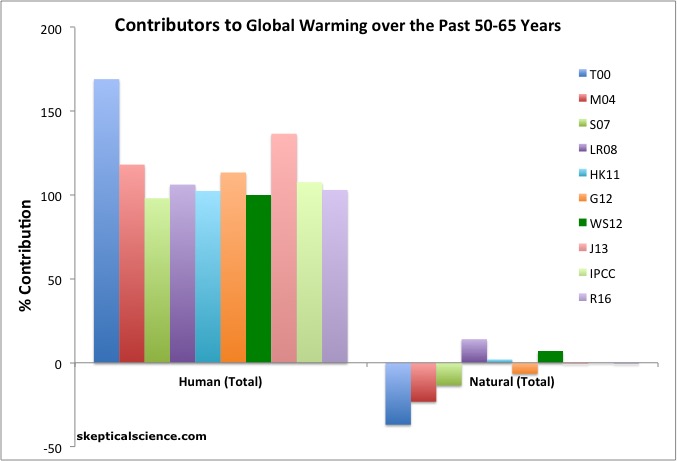
- This is the biggie. What is the attribution for the temperature trends in recent decades? The question doesn’t specify a time-scale, so let’s assume either the last 60 years or so (which corresponds to the period specifically addressed by the IPCC, or the whole difference between now and the ‘pre-industrial’ (say the decades around 1850) (differences as a function of baseline are minimal). For the period since 1950, all credible studies are in accord with the IPCC AR5 statement:
It is extremely likely that more than half of the observed increase in global average surface temperature from 1951 to 2010 was caused by the anthropogenic increase in greenhouse gas concentrations and other anthropogenic forcings together. The best estimate of the human-induced contribution to warming is similar to the observed warming over this period.
The US National Climate Assessment attribution statement is a bit more specific than the one in IPCC:
The likely range of the human contribution to the global mean temperature increase over the period 1951–2010 is 1.1° to 1.4°F (0.6° to 0.8°C), and the central estimate of the observed warming of 1.2°F (0.65°C) lies within this range (high confidence). This translates to a likely human contribution of 93%–123% of the observed 1951–2010 change. It is extremely likely that more than half of the global mean temperature increase since 1951 was caused by human influence on climate (high confidence). The likely contributions of natural forcing and internal variability to global temperature change over that period are minor (high confidence).
This summary graphic is useful:

Basically, all of the warming trend in the last ~60yrs is anthropogenic (a combination of greenhouse gases, aerosols, land use change, ozone etc.). To get a sense of the breakdown of that per contribution for the global mean temperature, and over a longer time-period, the Bloomberg data visualization, using data from GISS simulations is very useful.
The difference in the bottom line for attribution for the last ~160 years is that while there is more uncertainty (since aerosol and solar forcings are increasingly shaky that far back), the big picture isn’t any different. The best estimate of the anthropogenic contribution is close to the entire warming. The potential for a solar contribution is slightly higher (perhaps up to 10% assuming maximum estimates for the forcing and impacts). In all cases, the forcing from anthropogenic greenhouse gases alone is greater than the observed warming.

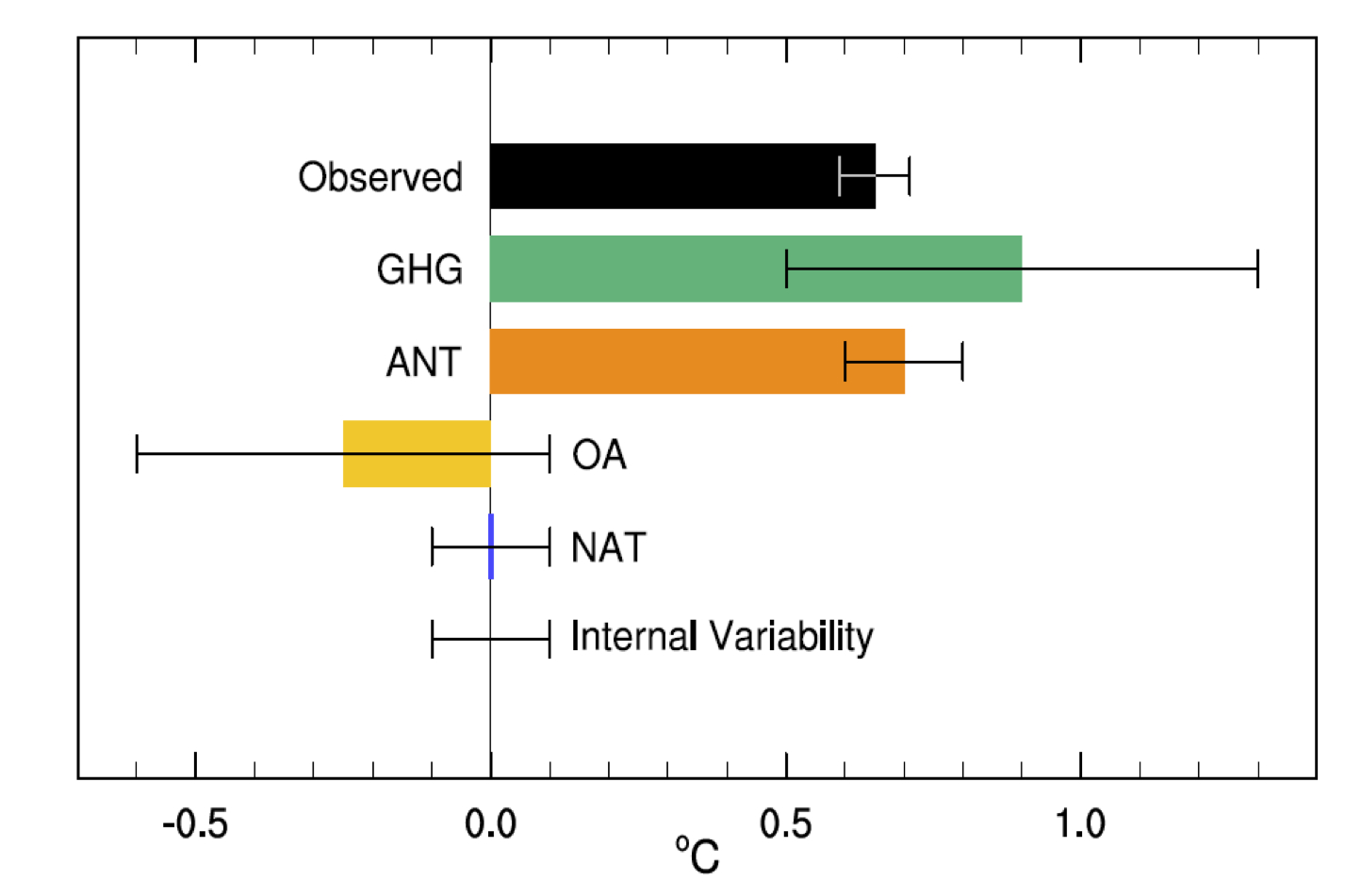
Figure 10.5 from IPCC. Assessed likely ranges (whiskers) and their mid-points (bars) for attributable warming trends over the 1951–2010 period due to well-mixed greenhouse gases (GHG), other anthropogenic forcings (OA) (mainly aerosols), natural forcings (NAT), combined anthropogenic forcings (ANT), and internal variability.
The role of internal climate variability gets smaller as the time-scale increases, but needs to be accounted for in these assessments. Note too that this can go both ways, internal variability might have wanted to cool overall in one period, and warm in another.
Prior to ~1750, atmospheric CO2 had been stable (within a few ppm) for millenia sustained by a balance between natural sources and sinks. This figure shows the changes seen in ice cores and the instrumental record.

References
- C.W. Snyder, "Evolution of global temperature over the past two million years", Nature, vol. 538, pp. 226-228, 2016. http://dx.doi.org/10.1038/nature19798
- J.D. Hays, J. Imbrie, and N.J. Shackleton, "Variations in the Earth's Orbit: Pacemaker of the Ice Ages", Science, vol. 194, pp. 1121-1132, 1976. http://dx.doi.org/10.1126/science.194.4270.1121
- G. Roe, "In defense of Milankovitch", Geophysical Research Letters, vol. 33, 2006. http://dx.doi.org/10.1029/2006GL027817
- P. Köhler, R. Bintanja, H. Fischer, F. Joos, R. Knutti, G. Lohmann, and V. Masson-Delmotte, "What caused Earth's temperature variations during the last 800,000 years? Data-based evidence on radiative forcing and constraints on climate sensitivity", Quaternary Science Reviews, vol. 29, pp. 129-145, 2010. http://dx.doi.org/10.1016/j.quascirev.2009.09.026
- M. Höpfner, M. Milz, S. Buehler, J. Orphal, and G. Stiller, "The natural greenhouse effect of atmospheric oxygen (O2) and nitrogen (N2)", Geophysical Research Letters, vol. 39, 2012. http://dx.doi.org/10.1029/2012GL051409
- R. Philipona, and B. Dürr, "Greenhouse forcing outweighs decreasing solar radiation driving rapid temperature rise over land", Geophysical Research Letters, vol. 31, 2004. http://dx.doi.org/10.1029/2004GL020937
- M. Etminan, G. Myhre, E.J. Highwood, and K.P. Shine, "Radiative forcing of carbon dioxide, methane, and nitrous oxide: A significant revision of the methane radiative forcing", Geophysical Research Letters, vol. 43, 2016. http://dx.doi.org/10.1002/2016GL071930


Mr. Know It All @41
“Nigelj, in the USA when you go to court you are judged according to the law, not what would be “just”,”
I never said anything about what would be “just”. I said the law of torts might apply, which is the law related to negligence. Or the law of criminal negligence may apply. Everyone owes a duty of care. You dont need any specific law related to CO2.
By analogy there is no law against selling tobacco, but the tobacoco companies agreed to pay billions in various federal lawsuits.
This current case regarding emissions appears to be based on similar principles.
Hello Gavin,
Thanks for the interesting post. Without seeming to be a bit churlish or dismissive of your effort (and condescending to the judge), some of your answers to the judge, if this is what you intended, will for the most part be way above his head. In my experience lawyers are, commonly, the most scientifically illiterate race of otherwise intelligent beings on the planet!! A lawyer’s training and thought processes are the very antithesis of scientific method. I would suggest that the judge’s need to ask some of the questions posed might to his credit reveal a willingness to learn, but I think also reveals a considerable misunderstanding of global warming science, and even a rather primitive thinking, that for instance human breathing might be an issue.
I am a general medical practitioner, with a long standing interest in science, and especially global warming, though the word “interest” is obviously inadequate, worry or deep concern would be more appropriate. As a general practitioner I am used to explaining sometimes complicated medical issues to patients, where their most complete understanding, within their capacity, has to be part of their successful management and treatment.
Question 1 What caused the various ice ages (including the “little ice age” and prolonged cool periods) and what caused the ice to melt? When they melted, by how much did sea level rise?
Answer. The postulated and very likely cause of the ice ages are variations of the earths orbit around the sun, and variations of the inclination of the earth’s axis in relation to this orbit, which cause variations of the sun’s heating of different parts of the earth at different times of the year (mainly the northern hemisphere) over long periods of time (tens of thousands of years). These are well described cycles, which have become known as Milankovitch cycles, after the name of the Russian scientist who for the first time in the 1920s was able to correlate these orbital and rotational variations (which other scientists had known about for many years) with the dates of various ice ages which had been more recently determined. You can read about this in Wikipedia https://en.wikipedia.org/wiki/Milankovitch_cycles – there is a very detailed explanation.
The so-called “Little Ice Age” was a cooling of the Northern Hemisphere climate (and possibly less markedly in the Southern Hemisphere) in the period of the fourteenth century to the the 1850’s, approximately. It came after a period of a relatively warm climate called the Mediaeval Warm Period. The cause of this relatively short lived cooling (it was not a true “ice age”) is not fully known, but the sun could have been cooler, there may have been more volcanic eruptions, there is a small but persistent cooling trend due to orbital cycles (as explained above).
Whilst this is the basic cause of the cyclical nature of the ice age and the warmer inter ice age climate of the earth in the last few million years at least, there are natural “feedbacks” in the earth which then exaggerate this climatic change. The most important in this is the release of CO2 from the warming oceans. As oceans warm, it can’t absorb as much CO2, which is then discharged into the atmosphere. CO2 is as you know, the most important global warming gas, as it stays in the atmosphere for long ages, this then is the major “amplifier” of a warming globe between the ice ages. Another important feedback is the reflection of light and heat by icy and snowy areas in the Arctic (and less so the Antarctic) region. This is called “albedo”. Snow acts like a mirror, and reflects up to 90% of the sun’s radiation back into space, sea ice about 50% whereas the open sea or ocean reflects just 6%. The open, ice-free ocean therefore is a great absorber of the sun’s radiation. There is a dramatic demonstration of this effect happening now as the Arctic ice retreats at an alarming rate, and the Arctic region warms twice as quickly as the rest of the planet. It is almost certain that the strange extreme weather patterns now observed throughout the northern hemisphere are related to this arctic warming and the consequent weakening of the jet streams that lie between the arctic and the more temperate northern lands. Other feedbacks include forests, and most importantly, water vapour, which as the temperature of the atmosphere rises increases in the atmosphere (think tropical rain forest), and water vapour is a potent greenhouse gas (but it is not the “controller” of our climate because it does not accumulate in the atmosphere, only gases like CO2, methane and nitrous oxide do this) See Skeptical Science https://skepticalscience.com/co2-lags-temperature.htm
At the height of the last ice age, sea levels were about 120 metres below present day levels, and the average rise of sea level during the return to our present climate was about 1 metre per one hundred years. During the last ice age, what is now known as Alaska was physically connected to eastern Siberia by land, which some call “Beringia”. You can find a map of where it was here https://en.wikipedia.org/wiki/Beringia#/media/File:BeringiaMap-NPSgov.jpg The IPCC agrees that a rise of one metre in sea level is possible by the end of this century. You can imagine just how serious that will be for those living in Florida, parts of China, Europe, S E Asia and Oceania.
Question 2 What is the molecular difference by which CO2 absorbs infrared radiation but oxygen and nitrogen do not?
Answer. This is very difficult to explain to a non-scientist, but basically it is an established scientific fact based on long-established experiment and theory that simple molecules like O2 and N2 don’t absorb infra-red radiation whereas more complicate molecules such as CO2 and H2O can. Wikipedia states that molecules containing two atoms of the same element, or gases such as Argon which have just one atom per molecule, have no net changes in their distribution of electrical charges when they vibrate. That’s difficult to understand. If you check on the internet you’ll find many differing explanations as due to “resonance”, “quantum mechanics” “bipolar molecules” etc etc. which to any layperson, however interested or intelligent, is mostly gobbledygook. When CO2 absorbs the radiation it “excites” the molecule, causing it to vibrate more energetically. This energy can then be transferred to any other gas molecule in the atmosphere causing it to heat up (that’s what heat is – the measure of molecular vibrational energy). This knowledge is not new; the same year as Charles Darwin published “The Origin of Species”, John Tyndall, an Irish scientist, published a paper in 1859 describing how he measured the absorption of infrared radiation in his laboratory, finding that CO2 and water vapour absorbed the radiation, whereas nitrogen and oxygen, the main gases in the atmosphere, do not. https://earthobservatory.nasa.gov/Features/Tyndall/ No scientist in the last 150 years has been able to demonstrate anything contrary. In addition one should point out that you need very little of these gases in the atmosphere to have such a profound effect. CO2 is measure in parts per million, for instance.
Question 4. Does CO2 in the atmosphere reflect any sunlight back into space such that the reflected sunlight never penetrates the atmosphere in the first place?
Answer – Basically no. CO2 does not “reflect” light and heat as far as this question is posed. But there is a small subtle effect that might cause a reduction of global warming from CO2 by a very small figure, perhaps 4%. So, basically, not an issue to be worth considering.
Question 5 Apart from CO2, what happens to the collective heat from tail pipe exhausts, engine radiators, and all other heat from combustion of fossil fuels? How, if at all, does this collective heat contribute to warming of the atmosphere?
Answer. All the heat of tail pipe exhausts, radiator and all other burning of fossil fuels whether in coal or gas burning electricity generation, home heating etc warms the atmosphere, a bit. That is true. The very large proviso though is that compared with the indirect effect of the CO2 warming the atmosphere, the figures are truly tiny. The Skeptical Science site refers to a paper by Flanner in 2009, a summary of which can be found here http://www.cgd.ucar.edu/tss/ahf/, that shows the direct heat from burning fossil fuels is just 1% of the effect of the CO2 produced by this burning on the absorption of heat by the atmosphere from the sun, i.e. global warming. Another way of looking at this is that the heating of the earth due to global warming is equivalent to the energy in four Hiroshima sized atom bombs exploding every second. You will obviously realise that no way are we burning anything like that amount of energy directly. It can be confusing working all this out as scientists are fond of using fundamental measures of heat or energy like peta Joules, whereas most of us are more likely to understand at BThU or Kilowatts etc.
Question 6 In grade school, many of us were taught that humans exhale CO2 but plants absorb CO2 and return oxygen to the air (keeping the carbon for fiber). Is this still valid? If so, why hasn’t plant life turned the higher levels of CO2 back into oxygen? Given the increase in human population on Earth (four billion), is human respiration a contributing factor to the buildup of CO2?
Answer. No. Not at all. Your query is directed at the biology of humans on this planet, but of course we also have billions of domestic animals that also excrete CO2. The answer is just a bit of applied logic. Where does the CO2 that we breathe out come from? From the carbon contained in the food we eat. This could be from animals or plants, but ultimately the carbon we eat comes eventually from plant sources that photosynthesise – extracting CO2 from the air to build up the more complicated organic materials and structures that make up plants. Thus every molecule of CO2 that we breathe out we are merely returning to the atmosphere what was taken from it very recently. The total effect is therefore neutral, and it doesn’t matter how many people or animals there are. However, note that the indirect effects of this mass of human population and their domesticated animals is massive. What is global warming, deforestation, environmental damage, soil loss, ocean acidification and depletion, but the effect of far too many human beings coming to the limits of the earth’s ability to provide for them or deal with the waste we produce? It is not human breathing that is the problem, but human breeding. Your question also brings up the subject of plants taking up more CO2 as its concentration in the air rises. This is true. Plants do take up some of this excess CO2 but other human practices such as agriculture and forest depletion counteract this, and additionally the effect is nowhere big enough to counter the massive amounts of fossil fuels we burn. Discussions found on the internet reveal just how complicated and multifactorial this is. The fact remains that CO2 is rising every year, and plant growth is not stopping this.
Question 7 What are the main sources of CO2 that account for the incremental buildup of CO2 in the atmosphere?
Answer. Here we’ll be mostly affirming what you probably already know. The main source of CO2 is burning fossil fuels. We burn a lot, geological quantities of the stuff. Coal – about 8 billion tonnes per annum, nearly half of which is burnt by China and 1 billion tonnes by the USA. (That’s a mountain of coal about 6 km round and one kilometre high – nearly four miles diameter and over 3,000 feet high) Oil – about 35 billion barrels per annum. (That’s enough oil burnt every three years to fill Crater Lake in Oregon) Gas – 3,550 billion cubic metres per annum – that’s 3,550 cu kilometres (That’s a gas holder the height of Mt Everest (over 8,000 metres) with a diameter about 140 kilometres . That’s a total of about 36 billion tonnes of CO2 or about 10 billion tonnes of carbon equivalent per annum. Much more than a tonne per person on average on the planet. An American, like you, will discharge on average about 16.1 tonnes of CO2 per annum, that’s about 4.3 tonnes of carbon. (The difference between CO2 and carbon is a constant source of confusion in many discussions about global warming – one tonne of carbon burnt equals 3.67 tonnes of CO2). CO2 is far and away the main driver of climate change, as your question implies, the problem being for the planet that once emitted it is very slow to go away, many hundreds or even thousands of years of heating effects. Other obvious sources of CO2 are cement and steel manufacture, forest loss or burning, which the USA is beginning to become too well aware of, and agricultural practices. The EPA has a page here https://www.epa.gov/ghgemissions/sources-greenhouse-gas-emissions with a nice summary of the experience of the USA. Don’t also forget the methane is a potent greenhouse gas, about half of which arises from fossil fuel extraction and the other half from agriculture. Volcanic emissions of CO2. are real, but represent a small fraction of what humans cause by fossil fuel burning (less than 1%) For instance, the volcanic eruptions in Iceland of 2010 caused disruption of flights around Europe. More CO2 was saved by the aircraft being grounded than was discharged in the atmosphere by the volcano. It is true that the total movement of CO2 in the planet from air to soil to forest to sea to life on Earth and back again is much greater than human emissions. But these natural cycles, the Carbon Cycle, have been in balance for at least 10,000 years, in the period called the holocene, the temperate climate in which mankind has thrived. It is this balance that has been disturbed by human activity burning enormous quantities of hitherto sequestrated carbon, now accumulating inexorably in our atmosphere, much as a fat person gains weight inexorably as long he disturbs the balance by eating more food then his energy output requires. The difference may be very small, just half an ounce a day is ten pound gain a year. Yet half an ounce of food is a very small part of a daily human diet.
Question 8 What are the main sources of heat that account for the incremental rise in temperature on Earth?
Answer. This is your hardest question to answer, as the question seems to presuppose their are other sources of heat that are warming up the earth other than global warming due to CO2, methane, nitrous oxide (from agriculture and fertilisers) and CFCs (chlorofluorocarbons, from refrigerants etc) accumulating in the atmosphere from mankind’s various activities. As far as any reputable scientist studying these matters is concerned, there aren’t any other sources of heat. The sun is obviously the only significant heat source for the earth, and it’s the accumulation of heat retaining gases in the atmosphere that is causing this heat imbalance. The sun’s actual heat output varies slightly in a cyclical way, with sunspot activity waxing and waning over an 11 year cycle, but despite careful measurement, that has been done for well over 100 years, there’s no significant long term change in the sun’s heat output. So we can exclude the sun itself for any rise in the Earth’s temperature. The Earth’s interior is hot. A very small fraction of this heat leaks to the surface, about one tenth of one watt per square metre. In comparison the amount of heat coming from the sun is 342 watts per square metre. In any case, no-one is suggesting that the planet’s core is getting hotter, which would be needed to actually make our planet warm; to the contrary, the planet will be very slowly cooling as the radioactive elements in the core providing this heat gradually lose their energy. Does heat come to the planet from space or cosmic rays? It’s difficult to find any article addressing this matter, but if any at all, it will be minuscule. Some scientists query whether cosmic rays may have an effect on global warming, but this is very contentious and not accepted by most.
It’s to be hope that these answers and some of the references given will be helpful to you. The answers are quite long, but they are couched as accurately as possible in everyday and understandable language, and illustrated by some analogies. The internet site, Real Climate, ( https://www.realclimate.org ) has informative articles written by experienced and well-regarded atmospheric scientists, but can be hard for non-scientists to understand. Skeptical Science (https://www.skepticalscience.com) has articles about every aspect of global warming you could think of, and some you couldn’t, explaining how the science works and skilfully and rationally debunking the global warming doubters’ or contrarians’ arguments.
You will be needing to examine so many other issues in this coming law suit. Many scientists will have some very strong opinions as to the possible culpability of oil and energy companies in regard to global warming, which will not be expressed here, but they might also point out to the culpability of other industries, agriculture, politicians and economists and not least, yourself, ourselves and the general public in living our energy intensive life-styles, in reaching this parlous state of affairs in regard to our very existence on this planet. There won’t be a concerned scientist who won’t be wishing you the very best in this case and your judgement; your applied wisdom is very much needed.
[Response: To be clear, I have no role in these proceedings, I’m just trying to be helpful! Thanks for your responses, I’ve added some of them above. – gavin]
DDS 39: “The SCIENCE is not “politicized”. ”
If that is true then Scientists are exceptionally virtuous people.
BPL: Talk about not getting the point.
KIA, #41–
“Found guilty,” no–but you sure can be “held responsible” for any harms that result, especially if you knew or should have known that said harms were likely or possible.
Our friend nigel is correct, in principle: in the US, as in New Zealand, if ‘spewing CO2’ causes specific damages to a party, then they have the right to sue to be made whole. They may also be able to claim punitive damages under some circumstances. For example, there is no specific statute about excessively hot coffee, but in one celebrated case, Stella Liebeck won damages of $640,000–the initial jury award had been nearly $3 million–and likely received more than that in a confidential pre-appeal settlement.
https://en.wikipedia.org/wiki/Liebeck_v._McDonald%27s_Restaurants
Recall the old chestnut, attributed to Will Rogers, that ‘Your freedom to swing your fist ends where my nose begins.’ Or, as a judge in a somewhat parallel case–the ‘children’s lawsuit’ against the federal government–put it:
Now that view has been in effect upheld by the Ninth Circuit on appeal, and the suit will be going forward. Here’s the chronology of that case:
https://www.ourchildrenstrust.org/us/federal-lawsuit/
That doesn’t mean it’s ultimately going to be a winning case; unlike Stella Liebeck’s coffee, the causal linkage between action and harm is lengthy, even diffuse. (On the other hand, while lots of folks blamed poor Stella for the suffering she experienced, it’s pretty hard to argue that the plaintiffs in the Children’s Trust case created the problem.) I suspect that it will probably still be in litigation after we’ve definitively missed (or maybe achieved) 2 C mitigation goals. But it is most definitely a case.
And it may be winnable. A parallel case in Holland succeeded already:
https://www.theguardian.com/environment/2015/jun/24/hague-climate-change-judgement-could-inspire-a-global-civil-movement
So there are solid legal grounds for the San Francisco case giving rise to the current thread.
But hey, don’t blame me–‘I’m just the messenger.’ Judges made the rulings.
Gavin,
Here is my answer to the first two questions. If you find them useful then I can provide answers for others. I have tried to produce answers which the judge can readily understand.
The first question:
can be broken down into five separate questions:
1a. What caused the various ice ages
There were at least five ice ages which included at least two Snowball Earths that happened over 500 million years ago.
There was a less severe ice age 300 million years ago, and the current ice age called the Pleistocene, began about 2 million years ago. (We are still in the Pleistocene ice age with ice sheets covering Antarctica and Greenland.
We do not know for sure what caused those, or even if they all had the same cause, but we suspect a combination of the arrangement of the continents and low concentrations of greenhouse gases i.e. carbon dioxide.
I think the various ice ages to which your honor is referring are what we call glaciations. They are periods during the Pleistocene ice age when large ice sheets covered North America and Western Europe.
These seem to be caused by changes in the Earth’s orbit around the Sun, made worse by the resulting increases and decreases in carbon dioxide.
1a1 what caused the “Little Ice Age”
The Little Ice Age was not even a glaciation. It is just a nickname for a cold period when the river Thames froze over during winter. Its cause is not known, but it partly coincided with the Maunder Minimum in sunspots.
1a2 what causes prolonged cool periods
Since the Little Ice Age, there have been cool periods following massive volcanic eruptions.
1b. what caused the ice to melt?
As explained above it seems to have been a combination of changes in the Earth’s orbit and an increase in carbon dioxide.
1c. When they melted, by how much did sea level rise?
Figure 1 in Foster, G. L. and Rohling, E. J. (2013) ‘Relationship between sea level and climate forcing by CO2 on geological timescales’, Proceedings of the National Academy of Sciences, vol. 110, no. 4, pp. 1209–1214 shows that sea level rose by approximately 120 meters, nearly 400 feet. It also shows that sea level and CO2 are closely related.
2. What is the molecular difference by which CO2 absorbs infrared radiation but oxygen and nitrogen do not?
The difference in the molecules is that N2 and O2 are formed with two idenical atoms, whereas CO2, H2O, and CH4 have two types of atoms. If the two atmaos in nitrogen and oxygen molecules are of different isotopes they two will act as greenhouse gases. A full description of how greenhouse gases operate involves quantum mechanics, about which it has been said that if you claim to understand it then you don’t!
I am concerned that the questions posed by the judge are largely irrelevant to the question of whether petroleum companies are damaging or will damage the plaintiffs. For example, how are the causes of past ice ages relevant to that question? That and other questions (like 6, 7 and 8) seem eerily close to bogus climate science denier talking points. Overall, the questions seem to focus on minutia rather than the big picture of how CO2 emissions warm the planet and the evidence supporting that. Clearly they indicate (at a minimum) a need for basic education on climate science and global warming. At worst, this judge is already biased that AGW is a fraud, despite only the weakest of understanding of the issue.
[Response: It’s hard to tell. A more charitable explanation might be a desire to get past nonsense talking points and have ppl starting from some common basis when it gets to the salient issues. – gavin]
‘Agreeing with the suggestion above — seriously, whoever’s presenting answers should have carefully read:
https://www.theverge.com/2017/10/19/16503076/oracle-vs-google-judge-william-alsup-interview-waymo-uber
Remember, you can look this stuff up. And should. Having a ham license enabled me to understand which of a photon’s electric and magnetic fields (which are at right angles to each other) can excite a molecular bond, as described in the infrared spec page I linked above. I’d never understood just what happens when a photon interacts with a bond, til I read that page.
And ‘oogle, ‘oogle, ‘oogle. You’ll find a lot, e.g.:
— N6VSB
I didn’t really want to create an ‘essay’ to answer Alsup #1, but famously ‘you can’t always get what you want.’
So here’s an expansion and restructuring of answer #1. Probably expansion is exactly not what’s needed, but maybe there’s something useful in there. Note that I’m assuming the extant figures would be kept; I think they are useful.
One must first distinguish between the so-called “Little Ice Age” and the true “Ice Ages”.
The former, despite considerable research, remains imprecisely defined with respect to both time and space; some researchers have seen it as regional rather than global, and some identify multiple cooling ‘pulses’ rather than one discrete event. Its effects were relatively modest: perhaps 1 Celsius degree of cooling (1.8 F), a fall in sea level of approximately 30 centimeters (1 foot), and marginal increases in sea ice and terrestrial glaciers as well as descents in European montane ‘snow lines’ of perhaps 100 meters.
[Online review of LIA sea level research: http://www.kwaad.net/SeaLevel-MiddleAges-LittleIceAge.html%5D
Multiple causal factors have been suggested for the LIA: insolation change due to orbital cycles; low solar activity; high volcanic activity; and reduced atmospheric CO2 due to forest regrowth following human population collapses (the “Black Death” in Europe and Asia, and Columbian contact in the Americas.)
[See, eg., the literature discussion here: https://journals.ametsoc.org/doi/full/10.1175/EI157.1%5D
By contrast, true Ice Ages drastically reshaped the planet, with much greater changes in global temperature, sea level, and ice extent. Although there have been at least five such “Ages” during Earth’s history, only the most recent–which, strictly, remains ongoing today–will be discussed, since it is by far the best characterized and understood.
The current “Ice Age” began roughly 2.8 million years ago, with the onset of what geologists call the Pleistocene Epoch. It consists of repeated cycles of prolonged cool, dry climatic conditions, termed ‘glaciations’, followed by briefer ‘interglacials’ during which conditions become warmer and moister. (Confusingly, the cycles of glaciations and interglacials are often colloquially termed “Ice Ages” themselves.) Our current climate, termed the Holocene Epoch, is one of these interglacials, and has been ongoing for over 11,000 years.
During the preceding glaciation (the LGM, or “Last Glacial Maximum”), global mean temperature was approximately 6 Celsius degrees cooler, sea levels were at least 120 meters lower than at present. Virtually all of what is now Canada, together with considerable portions of the northern US, was covered with an ice sheet several kilometers thick. Dust levels in the atmosphere were up to 25 times higher than present, reflective of much lower levels of precipitation in most places.
http://www.pik-potsdam.de/~stefan/Publications/Journals/schneider_etal_grl_2006.pdf
The cycles of glaciations and interglacials have varied in length, frequency, and intensity over the Pleistocene. In the 1970s researchers showed that these changes were highly correlated to changes in the variability of the Earth’s orbit – the so-called Milankovich cycles (Hays, Imbrie and Shackleton, 1976). More recently, the growth and collapse of the ice sheets has been specifically tied to the incoming solar radiation (insolation) at high latitudes (Roe, 2006).
However, several ‘feedbacks’ are known to have amplified the magnitude of the glacial cycles. These feedbacks may be broadly divided into two classes: shortwave forcings (which would include ice, dust, and vegetation) and longwave forcings (principally, greenhouse gases such as carbon dioxide.) Both types affect radiant energy, but in different frequency ranges.
Shortwave feedbacks are those which affect relatively high-frequency visible light reaching the Earth from the Sun. For example, if ice extent increases, the increase in reflective surface will mean that more sunlight is reflected back into space without ever having its energy absorbed into the Earth system. This will tend to decrease the temperature. Conversely, vegetation tends to be very absorptive of visible light, so increases in forest or grassland will tend to increase temperature.
Longwave feedback affects the lower frequency infrared light emitted by objects at Earthly temperatures–this often used to be termed ‘radiant heat’. More greenhouse gases in the atmosphere impede the escape of thermal infrared radiation to space, and thereby raise temperature.
These factors are termed ‘feedbacks’ because they both cause, and may themselves be caused by, changes in temperature. We may illustrate with a simple conceptual model of the transition to the present interglacial, as follows.
As temperatures warmed slightly due to insolation changes caused by orbital cycles, ice sheets retreated slightly. This brought a small additional warming, and allowed plant life to expand a bit polewards–yet another small warming influence. The ocean surface warmed a bit, too, releasing carbon dioxide to the atmosphere and strengthening the warming trend further, and as frozen ground thawed, the potent greenhouse gas methane would have been released as well, increasing the ‘longwave forcing’.
And with each increment of warming, ice would retreat further, vegetation would advance accordingly, and more greenhouse gases would be released from ocean waters or frozen ground. In this manner, warming would be, to a certain extent, self-reinforcing. (Corresponding processes would operate during cooling phases, too, though not in complete symmetry with the warming phases.)
[From the preview I see the formatting of the post is lost, making it harder to read. If there’s some way to maintain the indentations, please educate me. Otherwise I ask your patience.]
I agree with Thomas @ #8 that the format of the presentation of the material should be a tutorial that answers the Judge’s questions. I’m unsure from the intro whether the two sides get 2 hours each for presentation or 1 hr each for a total of 2 hrs. The more time the better, but you gotta live with what the Judge allows.
I recommend this outline. Liberally sprinkle with references.
I. Introduction
What occurs to me as I read the Judge’s questions is: What concept(s) does the Judge need to understand correctly in order to comprehend an answer (or, sometimes, to formulate the question reasonably)? The tutorial should provide the answer to that question (“Judge, here are the concepts you need to understand…”) and then show how the correct understanding addresses the questions (“…in order to understand the answers”).
Proceed along the lines that you need bricks before you can construct the building: illustrate the notion that climate science is based on deep historical roots, & draws support from many disciplines and lines of evidence.
It might help to have a q-n-a part to the presentation.
II. Basic concepts necessary to climate science
A. Concepts from physics
1. Understanding isotopes [each point having brief, historical “how we came to know this” section]
a. historical context of isotopes
b. stable vs radioactive
c. isotopes important to climate science [here, very general. Detail in part III below]
i. C isotopes
ii. O isotopes
iii. others?
[Details about how these bear on the Judge’s questions in part III below]
2. Spectroscopy, selective absorption & radiation of energy by certain elements
3. Fluid dynamics, atmosphere & ocean
4. Heat transfer
B. Concepts from chemistry
1. Carbon cycle & related matters
2. Breakdown of CO2, CH4 in the atmosphere
3. Atmosphere-ocean/atmosphere-land (weathering) chemistry
C. Concepts from astronomy
1. Orbital, axis orientation, precession variations historical development & importance
D. Concepts from geology
1. Ice ages, how “little ice age” is not an ice age in the same sense as Huronian, Cryogenian, etc
2. Paleoclimate, CO2
E. Plant biology
F. Oceanography? others?
III. How the above pieces address Judge’s questions
I get the feeling that the Judge has some misconceptions about things from the way some of the questions are phrased, particularly the question about atmospheric CO2 having reflective properties, and the “little ice age” being lumped together with other ice ages. Other questions, it seems to me, resolve rather easily when you understand relevant concepts (e.g., Carbon Cycle: how increased human population & exhalation of CO2 affects the increase of atmospheric CO2).
Re Q#3: The current answer “…emission from greenhouse gases … adds to the warming at the surface” is a true fact but is not a valid answer to the question of how the greenhouse effect alters surface temperatures (which underlies the judge’s query). A simple counterexample illustrates this: consider two massive cloud layers with equal shortwave albedo, one at high altitude, one at low altitude. The low cloud emits much more IR to the surface, but the high cloud produces much more surface warming.
Chris Colose (#44)’s answer is correct and a valid answer, but it’s a bit “opaque” for a non-technical audience. Here’s my version:
Q3: What is the mechanism by which infrared radiation trapped by CO2 in the atmosphere is turned into heat and finds its way back to sea level?
A3: The extra heat at sea level comes from the Sun. CO2 reduces the rate at which the atmosphere loses its energy to space via infrared radiation, which in turn reduces the flow of energy from the Earth’s surface to the atmosphere.
Energy from absorbed sunlight at sea level is transferred from the Earth’s surface to the atmosphere through direct heat exchange, evaporation, and exchange of infrared radiation. All three of these mechanisms slow down if the atmosphere is retaining extra energy, as it does if greenhouse gas concentrations increase.
The resulting accumulation of energy from the Sun raises temperatures throughout the climate system. A warmer atmosphere is able to lose more energy to space, so the whole climate system eventually approaches a warmer equilibrium.
@53
Thanks BPL. Saved me some typing.
Let me just add: When a football player plies his skilled profession on the field he just about never is acting politically. And even if there were a political thought it would have precious little outcome on the game.
When the same player takes a knee for the anthem he is acting politically.
There is a difference between the two situations that you are apparently blind to.
Perhaps Judge Alsup should apply for guidance to our new Secretary of State, who has strong views on global matters such as these.
I took a stab at reducing the IPCC 2013 report conclusions on ice ages down to something that any general audience should have little trouble with (I hope). The advantage of going this route in a courtroom, however, is that if detailed questions are asked or the summary is challenged, you can go right back to the IPCC report and to the individual scientific studies the report cites, it’s a direct correspondence.
Q1: What caused the various ice ages (including the “little ice age” and prolonged cool periods) and what caused the ice to melt? When they melted, by how much did sea level rise?
The timing and severity of ice ages are determined by two major factors, namely the level of sunlight falling on northern land masses and the associated levels of atmospheric greenhouse gases.
As the Earth orbits the sun, the gravitational tug of the other planets slightly alters orbital characteristics (precession, tilt, ellipticity) on a timescale of tens of thousands of years. These variations change the seasonal amount of sunlight falling on land surfaces in the northern and southern hemispheres.
Due to less sunlight falling on the northern hemispheres, snow fails to melt in the summer, accumulates and is compressed into glacial masses. These ice sheets have a higher albedo (reflectivity) and exert a local cooling effect. This in turn gradually causes carbon dioxide in the atmosphere to be removed and stored as land-based permafrost carbon or as deep-ocean CO2.
This gradual removal of CO2 from the atmosphere reduces the overall greenhouse effect and thus slowly draws the entire planet into an ice age, driving further ice sheet expansion over tens of thousands of years (a complete ice age cycle is around 100,000 years)
Many other factors influence regional climate variations such as the Little Ice Age, the Medieval Warm Period, and the Younger Dryas climate excursion. Periods of volcanism can cool the climate (as with the 1991 Pinatubo eruption), methane emissions from increased biological activity can warm the climate, and slight changes in solar output and orbital variations can all have climate effects which are much shorter in duration than the ice age cycles, ranging from less than a decade to a thousand years in duration (the Younger Dryas).
Ice sheets begin to retreat from their maximum extent due to the increase in sunlight driven by the overall orbital variations. As ice sheets retreat, several mechanisms cause greenhouse gases – carbon dioxide, methane and nitrous oxide – to increase. Melting permafrost outgasses CO2 and methane, and the decrease in sea ice allows oceanic CO2 to mix back into the atmosphere; taken together, these processes greatly amplify the effect of increased sunlight, driving a relatively rapid exodus from the ice age.
Polar amplification, in which temperatures at the poles rise more rapidly than temperatures at the equator (due to factors like the global atmospheric and oceanic circulation of heat from the equator to the poles), plays a major role in the rate of ice sheet retreat.
Ice sheet retreat continues until a new equilibrium temperature state is reached, one determined largely by the end-point of atmospheric CO2. Variations in atmospheric CO2 over the past million years range from 190 ppm at the height of ice extent, to 280 ppm at minimum ice extent. Methane variations have also been shown to have significant effects on shorter timescales (See Younger Dryas termination).
Melting of ice sheets over the end of the last glacial maximum resulted in 130 meters of sea level rise over a period of 13,000 years, an average rate of rise of 1 meter per century, although the rate may have varied widely.
Future forecasts of climate models forced with greenhouse gas levels as high as modern ones tend to result in Pliocene-like climate (~3 million years ago) when sea levels were estimated to be 14 meters higher than they are today. How long it would take this scenario to unfold is a matter of some controversy among climate scientists.
Alistair MacDonald @55
This isn’t true, for planet Earth at least, because Earth doesn’t emit significant IR radiation at the frequencies that these stretching vibrations occur. This is explained both in the original text that Gavin posted and my subsequent amendment.
They would, thus, be greenhouse gases for a significantly hotter planet, but strength of the IR absorption is governed by the change in dipole moment during the vibration and this coupled with the relative scarcity of the isotopes mean that their effect would be extremely weak.
Okay, one little nit-picky issue with Q2 is that O2 and N2 actually DO absorb infrared radiation, just at shorter wavelengths than matter for the Earth’s infrared emission spectrum (3-27 microns, with a peak around 9 microns or so). Some itchy ExxonMobil lawyer might bring that up so:
Q2: What is the molecular difference by which CO2 absorbs infrared radiation but oxygen and nitrogen do not?
This question is best answered by considering the characteristics of all the main infrared-absorbing gases in the atmosphere – carbon dioxide (CO2), water vapor (H2O), ozone (O3), methane (CH4), nitrous oxide (N2O), and the chlorofluorocarbons (CFCs). Notably, they all have at least three atoms per molecule, whereas the infrared-transparent gases, O2 and N2, have only two.
In terms of the physical processes involved in light absorption, many infrared photons have enough energy to excite vibrations in 3-atom (or more) molecules, but, for infrared radiation wavelengths emitted by the Earth, few such photons have enough energy to excite vibrations in 2-atom molecules.
While O2, for example, does absorb infrared radiation of about 1.0 micron, it is transparent to the longer-wavelength infrared that the Earth generates. The wavelength of photons emitted by a body is correlated to its temperature, via the blackbody approximation; the Earth’s emission peak is at about 9-10 microns, and only emits wavelengths between ~3 and ~27 microns. This is the reason O2 has little greenhouse effect, even though it does absorb shorter wavelengths of infrared light.
CO2, in contrast, strongly absorbs wavelengths >13 times longer than O2 does, as well as other bands around 2-3 and 4-5 microns, while water vapor absorbs strongly from around 5-8 microns. Thus, CO2 and H2O strongly absorb within the spectrum of infrared emitted by the Earth, as do the other greenhouse gases.
(The relationship between molecular structure and photon absorption is a quantum mechanical effect with no good commonplace everyday analogy; some have tried to represent this by visuals of springs stretching vs. bending but that’s something of a hand-waving explanation. The main issue is the (a)symmetry of the distribution of electrons within the molecule, which accounts for a 2-atom molecule, carbon monoxide, having an absorption band around 5 microns, for example. Detailed experiments and calculations over many decades have sorted this issue out, but it required quite a few physics PhDs to do so.)
Since each of the infrared-absorbing atmospheric gases has its own unique absorption spectrum, the total infrared absorption capacity of the atmosphere is then due to the real-time concentration and distribution of all the gases in the atmosphere, from the surface to the stratosphere.
An important factor is that atmospheric pressure strongly affects the absorption spectra of gases, a phenomenon called pressure broadening. As a result, computing the transfer of infrared radiation throughout the atmosphere, with variations in pressure, temperature and concentration of each gas type, is a challenging problem that was not well understood until the 1950s. One general result of these complexities is that CO2 has its strongest warming effect about 10-12 miles above the surface of the earth.
> quantum mechanics
Alsup holds an “Amateur Extra” ham radio license — highest level.
http://wireless2.fcc.gov/UlsApp/UlsSearch/license.jsp?licKey=722727
He will understand the answer I quoted from the infrared spectroscopy site above.
(I had no idea that the orientation of the molecule affected its ability to absorb energy — makes me wonder why a bond will emit a photon in a random direction. If only we could make the molecules aim their outgoing infrared up and away from the planet … but no.)
http://www.umsl.edu/~orglab/documents/IR/Image84.gif
DDS @39
It is the structure of the scientific method that absolutely forces scientists to conduct their investigations in a “virtuous” way. If they do not, they get pitilessly hammered by their peers. So, while the practitioners of the scientific method are no more virtuous than the rest of society, they are forced to be scrupulously honest in reporting their findings.
I think answer #3 is not very helpful as it stands; IMO, few people whose reasoning is oriented strongly toward the verbal and qualitative are going to make much sense of it, and presumably that would include a high proportion of jurists. I hacked out an interpretation for myself, but at nearly 800 words it’s probably too long as initially written. But here’s a more manageable cut-down version, finishing with Martin Vermeer’s Tyndall quote as a rhetorical flourish–and, like my previous effort, assuming that the extant diagram is retained:.
We can illustrate the mechanism of the greenhouse effect with a simple model. Since Earth’s atmosphere is mostly transparent to visible light, most solar energy (S) passes freely through the atmosphere and is absorbed at the Earth’s surface. There, it warms the ground or water, and from there energy (G) will be re-emitted to the atmosphere according to the Stefan-Boltzman Law:
G = σ Ts4
The symbol σ is the Greek letter sigma, which here stands for the Stefan-Boltzman Constant–a very small number which we do not need to investigate further at this time.
So emitted energy is proportional to the fourth power of the surface temperature (Ts).
As the atmosphere warms under the influence of G from the ground, it re-emits the energy received. But these atmospheric emissions are omnidirectional, so while half of the absorbed energy ends up escaping to space, half of it ends up back at the surface. Thus, Ts is a function not just of S and G, but also of downwelling ‘longwave’–that is, infrared–radiation from the atmosphere.
This brings us to the last undefined symbol in our diagram–the Greek letter lambda, λ. It is conventionally the symbol for “emissivity”–loosely, ‘the ability to emit radiant energy.’ In this case, it means the emissivity of the atmosphere–effectively, the strength of the greenhouse effect. It is given as a coefficient–a decimal fraction between one and zero, where a perfect reflector has an emissivity of zero, while a perfect emitter has an emissivity of one.*
One can see that, the higher the emissivity, the less energy is able to escape directly to space, and the more ‘longwave’ energy is received from atmosphere to ground. There are many more subtleties involved, but this essential point was almost immediately realized by John Tyndall in 1859-60, when he conducted a series of experiments measuring the infrared emissivity of various gases, including the two most important greenhouse gases, water vapor and carbon dioxide.
As he later put it, somewhat poetically:
As a dam built across a river causes a local deepening of the stream, so our atmosphere, thrown as a barrier across the terrestrial [infrared] rays, produces a local heightening of the temperature at the Earth’s surface.
*Note: This definition of emissivity may seem confusing; shouldn’t a ‘perfect reflector’ have an absorptivity of zero, not an emissivity of zero? The short answer is that it does–but the longer answer also notes that the absorptivity and emissivity of a given substance or object are directly related. For instance, a common formulation of Kirchoff’s Law of Thermal Radiation is this:
For an arbitrary body emitting and absorbing thermal radiation in thermodynamic equilibrium, the emissivity is equal to the absorptivity.
Robert Ingersol @56
The judge may be a climate sceptic, or just want information, or may be just be a naturally questioning sort of person, or the judge may want to be seen to be balanced and prepared to ask commonsense questions of both sides so he cant be accused of bias. Or it could well be as Gavin says.
However we probably will never know, so dont annoy the judge. Answer every question he asks nicely, clearly and in a way he will find comprehensible.
Respect his questions, whether you feel they are the right ones or not! You really dont want to antagonise the judge. Believe me I have been involved in court cases and I see what goes on.
Add any other material you think he might find useful, but as an appendix. The historical essay at post 30 would be useful to include.
#57, Hank Roberts–
OK, good catch. Clearly, Alsup is not afraid of numbers or tech!
Which also means that my response to #3 would probably come across as a bit condescending. Damn.
Eli’s on it:
http://rabett.blogspot.com/2018/03/dear-judge-alsop-spectroscopic-basis.html
@67
Unless you posit a worldwide conspiracy of thousands upon thousands of scientists with many different political and cultural orientations and views all coming together to comprise the most airtight conspiracy in the history of the world to support American liberal politics.
Which, of course, is a central tenet of denierism.
I’ve always wondered why, oh, Japanese scientists would be so driven to engage in a conspiracy to support American liberal politics.
51 and 54 – nigelj and Kevin
Still have the problem that there are no damages from AGW, thus no case. Much of what the 9th circuit rules is overturned – they’re known as the 9th circus court of the US.
In the case of tobacco and coffee there were damages so plaintiffs at least had a weak case; even though the damages were done by the plaintiffs with full knowledge of what they were doing by smoking and spilling hot coffee.
51 and 54 – nigelj and Kevin
What damages are the plaintiffs claiming in their case? Nothing in the article, or in any answers stating damages.
57 & 66 – Hank Roberts
That google case is complicated. Good thing Alsup knows something about programming! His science knowledge may help in the CC case as well. I’ll bet he’s been studying up on CC – I think many folks are trying to actually understand it and not just accept the word of others – many of whom have a political agenda.
I thought HAM extra licenses had a 4 digit call sign? Maybe if you liked the 5 digit one you can keep it – can’t remember.
His wilderness protocol sounds good. Good idea to take a repeater directory with you or write down all the info for the repeaters you might be able to hit in the back-country; better yet, program them into your radio so you can scan for traffic. Good antenna is helpful – I’d think minimum for 2 meter would be 5/8 wave in wilderness, a directional antenna would be good too, although not easy to find one you want to take backpacking.
In the past, some HAMs used 10 meter DX to get a signal out of remote areas – I think they use a long wire antenna. Only problem is your signal may be heard anywhere in the world – just have to hope they can help you.
Phil @64,
I should have written, “If the two atoms in nitrogen and oxygen molecules are of different isotopes they too will act as greenhouse gases.”
My point is that to act as a greenhouse gas, a molecule must be IR-active. This requires that it has one or more interatomic bonds whose dipole changes with vibration. This does not occur with homogeneous diatomic molecules unless they are formed from two isotopes.
Note, that the frequency at which the absorption occurs is not the frequency of the vibration. The vibrational frequencies are quantised, and radiation is absorbed when the photon causes a change in the vibrational frequency.
It is more complicated than that, but since the judge only wants to know why O2 and N2 do not absorb IR radiation I thought what I wrote originally was sufficient.
On pages 458-9 of Chapter 9, VIBRATIONAL SPECTROSCOPY of Elements of physical chemistry 5th Ed. by Paula and Atkins, they write:
which backs up what I wrote.
This gets to the heart of the lawsuit. I strongly suggest you find a way to incorporate this info, or info like it.
60% more deaths…?
https://www.msn.com/en-au/news/australia/comment-the-temperature-is-rising-and-so-is-the-death-toll/ar-BBKdX3t?ocid=News
While what’s said about Point 2 and the nature of GHGs is true, I wonder why the main reason of N2 and O2 not being possibly GHGs and not being IR active is not even first of all clearly mentioned.
The reason is of course that neither their single stretching vibrational mode nor their rotational motion can strongly couple to the radiation field for simple symmetry reasons. With a single bond between identical atoms there can be no permanent dipole coupling radiation and rotation nor can there be an oscillating dipole coupling radiation and vibration.
Hence homonuclear diatomic molecules can only couple very very very weakly to IR radiation at a higher order multipole level namely quadrupolar or magnetic dipolar, typically say 10^-8 times less intense than coupling with heteronuclear diatomic molecules such as NO or CO at dipolar level.
Actually N2 and O2 may emit or absorb a sizable amount of IR radiation when in a gas at high pressure or density because of the frequent collisions that then destroy their mirror plane symmetry during a sizable fraction of a molecule’s trajectory. https://en.wikipedia.org/wiki/Collision-induced_absorption_and_emission
KIA 73: Still have the problem that there are no damages from AGW
BPL: Who says there are no damages? You?
Alastair @76
I don’t disagree with what you say (it is, by and large, standard undergraduate study). However, in @54 I did draw a distinction between gases that absorb IR and gases that are greenhouse gases which you appeared to have overlooked in your response.
The point is that although selection rules will determine whether a molecular vibration can absorb IR radiation, and is obviously necessary for a complete discussion of the issue, the issue can be simply addressed by consideration of the frequencies of absorption by the gas and emission by the planet. This is simply an issue of elegance of communication, and its quite possible for two people to hold different views on it. From my perspective, the mismatch in IR frequencies is sufficient reason to preclude O2/N2 from being greenhouse gases, and therefore a discussion on selection rules can be avoided, especially since someone will invariably pop up with the isotopically variant species red herring.
In addition the argument about mismatched frequencies is, I think, an intuitively obvious point.
If you’re interested in why these (isotopically variant) species are insignificant greenhouse gases, even for higher temperature planets, please see the section “The Origin of Peak Intensities” here https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry_Textbook_Maps/Map%3A_Organic_Chemistry_(Bruice)/13%3A_Mass_Spectrometry%2C_Infrared_Spectroscopy%2C_and_Ultraviolet%2F%2FVisible_Spectroscopy/13.10%3A_The_Intensity_of_Absorption_Bands where it describes the effect of the change in dipole moment on the intensity of the absorption. I’m sure this is probably in at least one of PW Atkins books, although, given your reference, I suspect my editions are somewhat older than yours.
re:73. “Still have the problem that there are no damages from AGW, thus no case.”
Patently false. For example, read “Explaining the Extreme Events of 2016 From a Climate Perspective”, Special Supplement to the Bulletin of the American Meteorological Society, vol. 99, No. 1, January 2018. The role of climate change is specifically addressed. In fact, as a good example of objectivity, there are some events where the climate change signal is not evident.
There’s that pesky scientific method including peer-review biting you in the arse once again.
Good catch by Hank R in #57. The judge should be able to handle technical concepts, with some work. All the more striking that his list of questions is *extremely* naive. Not in any sense of being dumb, just of not knowing the first thing about the subject, while separating wheat and chaff. If the judge has any actual judgement, he would forthrightly acknowledge this. He’s probably about where I was circa 15-20 years ago. Heard of it, hoped the right people were on it, but not deeply aware of the basics + consequences.
The attempts at direct answers are laudable, but my advice to someone in that position who wants to learn would be to take some hours to read Wikipedia on Global Warming, and read this blog starting at the beginning. Skip most or all of the comments, though for a deep dive the RC folks’ answers can be worth it. Being up to the minute on research is not the main point. Skimming and wading through RC will give an excellent sense of the basics, and of who can back up things, and who is just carping and distracting.
Mr. KIA:
“Much of what the 9th circuit rules is overturned – they’re known as the 9th circus court of the US.”
1. Alsup is a District Judge
2. Overturn rate of those heard by SCOTUS – 9th is only the third-highest of the ten circuits.
6th Circuit – 87 percent;
11th Circuit – 85 percent;
9th Circuit – 79 percent;
3. From March 2014 – March 2015, the 9th heard about 12,000 cases. 11 were overturned by SCOTUS. “Much of what the 9th circuit rules is overturned” is only true for ummm “interesting” definitions of the word “much”.
As is typical of right-wing smears, the facts don’t seem to back up the claims.
They do, just much much less absorbtion than CO2 because there’s no polar bond that would interact with the electrical field of the infrared photons.
Er, where’s Eli? I don’t know if what I’m finding is reliable or not, just poking around.
————-
O2 does absorb UV radiation, because _______
Also from http://onlinelibrary.wiley.com/doi/10.1029/2012GL051409/pdf
Geophysical Research Letters
Volume 39, Issue 10, Version of Record online: 24 MAY 2012
[Response: Interesting find! I’ll link this in above. – gavin]
#52 John Monro – exactly! A conversational review of fundamental science in units a lay person can understand. No “orders of magnitude”, no lambda symbols.
MR KIA @73-74
Regarding the basis of the legal case, please just read the links in the article above. In summary, the original case was bought under state nuisance law, which is tort law as I have explained twice now. And which KM has explained in considerable detail. It appears their goal is to bankrupt the companies rather the damages, but this is basically a detail.
Now the case has been moved to federal court to be heard under a federal common law nuisance statute (law of torts). The EPA law on CO2 emissions doesn’t apply in this case. I don’t know what remedy or damages they are seeking, because the links didn’t say, but the case is clearly going to being heard. Obviously they will be wanting something and the law will have provisions for some form of remedy, because that’s what laws do.
You are tilting at windmills!
I don’t know if the presentation will be done by climate scientists or by lawyers.
If the latter, you need to educate the lawyers first. This may help a bit — more, old and simplified, on electromagnetic waves and simple dipole radio antennas:
http://www.dtic.mil/dtic/tr/fulltext/u2/684938.pdf
Interestingly, the O2 molecule’s absorption in the near-infrared , ~1 micron, is forbidden – it’s a very weak absorption (that actually might affect the absorption of solar radiation by a small amount). So why does it show up???
Working backwards through the literature. . .
Details on the infrared selection rules (modern astrophysics textbook)
http://astro1.panet.utoledo.edu/~ljc/LectF.pdf
“Homonuclear diatomic molecules such as molecular hydrogen, nitrogen, and oxygen have no net dipole moment. As the molecule stretches and compresses itself in its one vibrational mode, the symmetry is unchanged, and at no time does a dipole moment exist. Thus, infrared transitions are truly ‘forbidden’, since symmetry requires that the molecular dipole moment can never vary from zero.”
But. . .
“Near-infrared absorption cross sections and integrated absorption intensities of molecular oxygen” (2000, Smith & Newnham)
http://onlinelibrary.wiley.com/doi/10.1029/1999JD901171/full
Which references . . .
“Absolute Intensities of the Discrete and Continuous Absorption Bands of Oxygen Gas at 1.26 and 1.065 Micron” (1965, Badger et al.)
https://authors.library.caltech.edu/10448/1/BADjcp65.pdf
“Molecular oxygen (O2) has absorption bands throughout the spectrum from the infrared (IR) to the ultraviolet . . . The oxygen absorptions at 1.06 and 1.27 um may be attributed to two types of absorption (1) from individual O2 molecules and (2) from O2 molecules that are involved in some interaction through collisions or transient pairings with other molecules (in this case either O2 or N2).”
And going back further:
Forbidden Transitions in Diatomic Molecules (1939)
Herzberg, G. Astrophysical Journal, vol. 89, p.288
“There are essentially three possible reasons for the appearance of forbidden transitions, that is, transitions which violate the ordinary selection rules. . .
. . .b) Some selection rules hold rigorously for electric dipole radiation; but transitions violating them may occur, though extremely weak, owing to quadrupole or magnetic dipole radiation. Examples of magnetic dipole radiation are the well-known atmospheric absorption bands of oxygen in the red . . . and the new infrared atmospheric absorption band of O2, at 1.27 microns.”
Finally! Actually the judge asked a slightly incorrect question (probably unwise to correct him publicly), the question should be, ”What is the molecular difference by which CO2 absorbs infrared radiation emitted from the Earth’s surface but oxygen and nitrogen do not?”
Cleverly, the judge has limited all this to a one-hour presentation from both sides. Otherwise it could take weeks.
I would still like to see the acronyms in the final picture replaced by the equivalent text. It would not be difficult and it would make the picture self-explanatory, with no need to consult the caption for the meanings.
https://www.nature.com/articles/35000166
A single-photon detector in the far-infrared range
S. Komiyama, O. Astafiev, V. Antonov, T. Kutsuwa & H. Hirai
Nature volume 403, pages 405–407 (27 January 2000)
doi:10.1038/35000166
It does not matter how clever your answers to the judge’s questions are.
The simple truth is that the oil companies did NOT use the fossil fuels to produce CO2.
It was people (warmists and non-warmists), who used the fossil fuels to produce CO2.
In general, non-warmists accept responsibility for their use of fossil fuels. However, warmists are strongly in denial. They blame it all on the oil companies.
KIA, #73 & 4–
And that would be ‘Exhibit A’ in the case that you are an ‘impacts denier’–no offense, I add punningly. Still no specific statute against it…
But addressing the merits of your statement:
http://www.tampabay.com/news/publicsafety/Hurricanes-wildfires-made-2017-the-most-costly-U-S-disaster-year-on-record_164310581
https://www.nytimes.com/2015/03/03/science/earth/study-links-syria-conflict-to-drought-caused-by-climate-change.html
https://daraint.org/wp-content/uploads/2012/09/EXECUTIVE-AND-TECHNICAL-SUMMARY.pdf
The gold standard, however, remains the comprehensive literature reviews periodically carried out by the IPCC–the Assessment Reports essentially summarize and synthesize the professional literature (which is where the true ‘scientific debate’ takes place). Working Group II is tasked with examining issues relating to impacts and adaptation. Here’s their ‘executive summary’–SPM–from the latest report (AR5).
https://www.ipcc.ch/pdf/assessment-report/ar5/wg2/ar5_wgII_spm_en.pdf
(NB–Summaries for Policy Makers have been historically attacked as vulnerable to politically-motivated spin from national governments, who must sign off on SPM wording, though they cannot alter content per se. However, such spin has most typically been exercised by oil-producing states (eg., Saudi Arabia) to underplay the seriousness and urgency of the problem.)
Some illustrative excerpts:
NB–“affecting water resources in terms of quantity and quality” means “damaging water resources”. That’s a specific harm, and it’s one accruing in the US Southwest–several jurisdictions have already spent billions of dollars on water supply measures to keep up with the drying observed to date.
Historically, the US (like many nations) has placed a high value on avoiding species extinctions (cf., ‘snail darter’ and ‘spotted owl’ controversies, not to mention the very existence of the Endangered Species Act.) Therefore, the loss of even a few species to ‘AGW’ constitutes a ‘harm’, and further the strong expectation of much larger future losses must be considered.
IOW, climate change is already episodically inducing food insecurity or worse in some places.
There is of course more, but I’m going to stop because I realize that while all this is good evidence for harms caused by anthropogenic climate change in general, to be really relevant to the topic at hand, we should be looking at the specific harms alleged by the plaintiffs in the suit over which judge Alsup is presiding.
https://www.sfcityattorney.org/2017/09/19/san-francisco-oakland-sue-top-five-oil-gas-companies-costs-climate-change/
So they are highlighting future damages, which will be vastly greater, while holding their cards close to the vest on damages already incurred. Stay tuned for trial, as the cities will undoubtedly produce evidence.
Dang, I sure wish I could edit those botched ‘end blockquote’ tags in the last two comments I made. Oh, well… sorry ’bout that. I’ll try to improve.
Note to self: scroll down and read before hitting ‘submit’!
Looking at the question of present climate harms in the Bay area, there’s not that much–though there’s ample evidence to support a high probability of extremely high future costs. For example, sez here that a 100-year storm could cost the area $10 billion, according to a study by the Bay Area Economic Council:
https://www.mercurynews.com/2015/08/13/flood-protection-and-preparing-for-sea-level-rise/
They are trying to restore coastal wetlands in the South Bay area to mitigate future storm damage, and spending money to do so (it’s not clear from this link how much.) And that’s not SF or Oakland, of course.
It’s also notable that tidal nuisance flooding occurs now at so-called King Tides, and is being used as a visualization tool for the future. It can also happen due to storm surge, as the ‘headline video’ in this link illustrates for the Embarcadero:
https://www.sfgate.com/bayarea/article/Bay-Area-coastal-flood-warning-issued-with-king-12402568.php
Given that there’s really no ‘if’ about future sea level rise (unless one is prepared to believe in magic), the apprehension about future costs is certainly understandable.
I also note that SLR is not the only flood risk potentially related to climate change which could affect the Bay area. I never heard about this $100 million event–which affected 14,000 people, and pretty much ruined the quality of life for some–til I did the search just now, but the lawsuits are already flying in San Jose, down at the southern tip of the bay:
https://www.eastbaytimes.com/2018/02/17/a-year-after-devastating-coyote-creek-floods-some-victims-still-struggling/
One can’t say from the story whether this is definitely related to climate change in any way–I can’t even tell from this link whether it is attributable to precipitation (though the timing is certainly suggestive). But it’s possible, and even if this particular disaster is not attributable to climate change, it still exemplifies very well what the risks from extreme precipitation ‘look like’, in the Bay area and around the world. It certainly reminds me that people here in South Carolina are still struggling to recover from Hurricane Matthew’s extreme precipitation last year, and from the extreme precipitation events of 2015.
> sea level rise
Don’t you wish the city lawyers would put Richard Alley on the stand for 20 minutes to address that question?
# 2. Re; #2 – I myself was long and simmeringly stumped as to why a ‘symmetrical’ molecule, such as Two heavy Oxygens, when welded in syzygy with a central smaller Carbon pivot, could inspire a “Dipole Moment.” After all, that molecule is like an old Studebaker: coming and going, it looks the same. In fact, it is symmetrical about its Pitch, Roll, and Yaw Axes, so what gives? When Doc Archer covered this @ his U. of Chicago MOOC, he held his arms up., fists doubling as the ‘Oxies,’ & head as the Pivot-Carbon, and proceeded to mimic the Funky Chicken.
That done it! I got it in a nanosecond.
Perhaps in addition to graphics, some presentation whiz can snip in ten seconds of video—just a thought. Or a brief cartoon could be fashioned to depict Just How, quantum mechanically, a passing electro-negative ‘wave-field’ disturbance, a momentary peak of traveling electro-negativity say, might, passing the CO2 IR ACTOR @ the speed of light, encounter a momentarily BENT molecule, W/a dipole acting similar to Water’s 104.45° bond & bend angle, tho not as severely bent. A similar cartoon might be crafted to show why (or how) the EQUIVALENT dumbbell bulk Oxygens and Nitrogens, wave-cancel, and thus Fail to Interact W/passing Infrared waves, or photons.
* [Incidentally, the Judge might benefit by the ‘Hand Hold,’ provided via the STORY of Joseph Fourier, generally credited as the ‘Discoverer’ of the “Greenhouse Effect”. While travelling for his first visit ever to an arid zone (Egypt’s Deserts, W/Napoleon), Fourier readily could FEEL, the rapidly chilling evening air, which contrasted so with his prior experiences in moist France. Nearly All Humans have experienced directly, hot and lingering humid discomfort–heat, which, when trapped by H2O vapors, lingers long into the late-night’s air, in say, Foggy Bottom, Washington D.C. So different from Phoenix or Vegas.]
You can find a lot just by looking, e.g.:
http://www.jonesday.com/files/Publication/7c1e79b0-c978-4a39-820a-fe9b7ea81dd8/Presentation/PublicationAttachment/a8a11055-68f1-4372-8895-07f0f920e94b/Who_should_do.pdf
Point being, often firms put a grayhaired elder partner up to present an argument that was crafted by young junior lawyers who have become intimately familiar with the details of the argument, which the elder partner may not fully understand. Judges are aware of this.
Oh, here’s the search that turned up that bit; using /Tools/Verbatim, which can help or hurt depending on your search terms chosen:
https://www.google.com/search?q=%22Judge+Alsup%22+presentation+written+oral&client=firefox-b-1-ab&source=lnt&tbs=li:1&sa=X&ved=0ahUKEwj_l8uq7vHZAhVC5GMKHUyfD-UQpwUIHw&biw=1348&bih=853
I’d close that parenthetical after the word “energies” and add a note about those higher energies:
… so they absorb only at higher energies). The higher energy ultraviolet photons, which can be absorbed by O2 molecules in the stratosphere, break that oxygen-oxygen bond and the freed oxygen can combine with O2 to make ozone (O3). The bonds in ozone, likewise, can absorb ultraviolet.
or something like that
“It is the structure of the scientific method that absolutely forces scientists to conduct their investigations in a “virtuous” way.”
Wow, who else here believes that?
Is that a common belief here at RealClimate?