Control of methane, soot, and other short-lived climate-forcing agents has often been described as a cheap way to "buy time" to get carbon dioxide emissions under control. But is it really?
Expectations for the outcome of the Cancun climate talks seem to be running low, and the suggestion has emerged that maybe we should forget about controlling CO2 emissions for now, and instead do something with short lived climate forcing agents like methane or soot. This is often described as "buying time" to put CO2 emissions controls into place. For example, in a recent New York Times Op-Ed, Ramanathan and Victor write:
"Reducing soot and the other short-lived pollutants would not stop global warming, but it would buy time, perhaps a few decades, for the world to put in place more costly efforts to regulate carbon dioxide." — Ramanathan and Victor
The idea that aggressive early action to control short-lived climate forcing "buys time" to do something about CO2 has often been pushed in the past, e.g. in various newsletters and press releases associated with the UNEP Atmospheric Brown Cloud program, for example
"The BC reduction proposal is not proposed as an alternative to CO2 reduction. At best, it is a short term measure to buy a decade or two of time for implementing CO2 emission reduction strategies." — Ramanathan, writing in the UNEP Black Carbon Newsletter.
To be fair, it should be acknowledged that such pleas for more attention to short-lived climate forcing are almost invariably accompanied by a salutary reminder that it is really CO2 that needs to be gotten under control, as in the quote above. Achim Steiner, writing in the same issue of the Black Carbon Newsletter writes "Paying attention to black carbon should not distract people from the real issue at hand, carbon dioxide." A similar sentiment is expressed in the Ramanathan and Victor op-ed. While emphasizing the central importance of CO2, Penner et al. argue that "…to provide short-term relief from climate warming, the short-lived compounds that induce warming need to be brought under control within a timescale of a few decades." (They also make the intriguing suggestion that doing so might provide a global experiment that could help constrain climate sensitivity.) Writing in Science, Stacy Jackson concludes that "… a focus on CO2 may prove ineffective in the near term without comparable attention to pollutants with shorter lifetimes"
All of this is well-intentioned stuff, none of it denies the central importance of CO2, and I’m sure there are many benefits to be had from reducing soot emissions sooner rather than later. Given the large agricultural component of methane emissions, keeping these emissions from growing in the face of a the need to feed a growing number of people is a serious challenge that must ultimately be met. But still, these proposals tend to convey the impression that dealing with the short-lived forcings now will in some way make it easier to deal with CO2 later, and that’s wrong. In this post, I will explain why.
To get a feel for the issues in play, we’ll first take a look at methane vs CO2. This provides a clean example, because methane has a straightforward, well-characterized warming effect which is easy to compare with that of CO2. If you’re just looking at the concentration of methane and CO2 at a given time, the methane/ CO2 equivalence is pretty easy to figure, since you can turn them both into the common currency of top-of-atmosphere radiative forcing. For example, doubling CO2 from 300 ppm to 600 ppm yields a clear-sky radiative forcing of 4.5 W/m2. Doubling methane from 1ppm to 2 ppm yields a radiative forcing of 0.8 W/m2, but since we started from such a low concentration of methane, it takes many fewer molecules of methane to double methane than to double CO2. Per molecule added, methane yields about 54 times as much radiative forcing as CO2. Note that most of this effect has nothing much to do with any special property of methane, but arises simply because the radiative forcing for most greenhouse gases is logarithmic in concentration, so you sort of get the same radiative forcing for everybody upon doubling their concentration — but if you start with somebody whose concentration is low, it takes many fewer molecules to double. That means that the CO2 equivalent of methane depends on what concentration you are starting with. If you started from a concentration of 10ppm, then the equivalence factor drops to 10. If you start out with equal amounts of methane and CO2 (300 ppm), then the equivalence factor drops further to 0.5. In that sense, methane is, intrinsically speaking, a worse greenhouse gas than CO2, though the crossover is at values that are so high they are only relevant (at most) to the Early Earth. ( I ran these calculations with the Python interface to the NCAR radiation model, provided in the Chapter 4 scripts of my book, Principles of Planetary Climate. They are done using an idealized clear-sky atmospheric profile, so the numbers are a bit different from what you’ll find in the IPCC reports, but it’s nice to have a calculation simple enough you can re-do it yourself.)
Things get a lot trickier when you try to bring time into the problem, because methane and CO2 have vastly different atmospheric lifetimes. Methane oxidizes to CO2 in about 10 years, and since we are dealing with so little methane, that extra ppm of CO2 you get after it oxidizes adds little ongoing warming. That means that the methane concentration in the atmosphere is determined by the methane emission rate averaged over the previous ten years, and the methane component of warming disappears quickly after emissions cease. In contrast, about half of CO2 emitted disappears into the ocean fairly quickly, while the other half stays in the atmosphere for thousands of years. Therefore, the atmospheric burden of CO2 in any given year is determined by the cumulative emissions going back to the beginning of the Industrial Revolution, and the warming persists for thousands of years after emissions cease. Over the long term, CO2 accumulates in the atmosphere, like mercury in the body of a fish, whereas methane does not. For this reason, it is the CO2 emissions, and the CO2 emissions alone, that determine the climate that humanity will need to live with for a time that stretches into the future at least as long as the time since the founding of the first Sumerian cities stretches into the past. The usual wimpy statement that CO2 stays in the air for "centuries" doesn’t begin to convey the far-reaching consequences of the amount of CO2 we decide to pump out in the coming several decades.
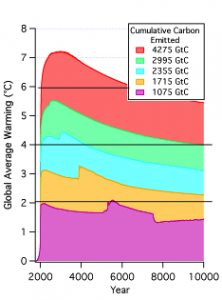
As a reminder of that, here’s a graph from the NRC Climate Stabilization Targets report (of which I was an author) summarizing how cumulative carbon emissions set the climate thermostat for the next 8000 years and more.
The numbers on each curve gives the total cumulative carbon emissions (in gigatonnes) during the time when human activities continue to emit carbon. These results are based on calculations by Eby et al using the UVIC coupled carbon/climate model, and they are really just a reprise of what Dave Archer has been telling all of us for years (e.g here, here and here). It turns out that it matters little to temperature whether all the CO2 is emitted in a carbon orgy near the beginning of the fossil fuel era, or spread out over a few hundred years. It’s cumulative carbon that counts, and pretty much it is the only thing that counts. A cumulative emission of a trillion tonnes of carbon just might keep the Earth below a warming of 2ºC, in line with earlier estimates equating the European Union target warming threshold with cumulative carbon (see our Trillion Tonne post). The peak warming scales approximately linearly with cumulative emissions, and the warming you get at the peak is pretty nearly the warming you are stuck with for the next millennium, with only slight declines beyond that. We are currently about halfway to our first trillion tonnes, but given the miracles of exponential growth, we are going to get there pretty quickly if nothing changes. If you go beyond, and dump 2355 gigatonnes into the atmosphere before kicking the fossil fuel habit, then the global mean temperature will still be 3ºC warmer than pre-industrial in the year 8000. That gives plenty of time for bad stuff to happen, including deglaciation of Greenland, loss of the West Antarctic Ice Sheet, or a destabilizing PETM-type soil carbon release. Note further that these calculations were done with a model designed to have a climate sensitivity similar to the IPCC median. Therefore, even if you hold the line at a trillion tonnes, there is still about a 50% chance that warming will exceed 2ºC.
Let’s suppose, however, that we decide to go all-out on methane, and not do anything serious about CO2 for another 30 years. To keep the example simple, we’ll think of a world in which methane and CO2 are the only anthropogenic climate forcing agents. Suppose we are outrageously successful, and knock down anthropogenic methane emissions to zero, which would knock back atmospheric methane to a pre-industrial concentration of around 0.8 ppm. This yields a one-time reduction of radiative forcing of about 0.9W/m2. Because we’re dealing with fairly short-term influences which haven’t had time to involve the deep ocean, we translate this into a cooling using the median transient climate sensitivity from Table 3.1 in the NRC Climate Stabilization Targets report, rather than the higher equilibrium sensitivity. This gives us a one-time cooling of 0.4ºC. The notion of "buying time" comes from the idea that by taking out this increment of warming, you can go on emitting CO2 for longer before hitting a 2 degree danger threshold. The problem is that, once you hit that threshold with CO2, you are stuck there essentially forever, since you can’t "unemit" the CO2 with any known scalable economically feasible technology.
While we are "buying" (or frittering away) time dealing with methane, fossil-fuel CO2 emission rate, and hence cumulative emissions, continue rising at the rate of 3% per year, as they have done since 1900. By 2040, we have put another 573 gigatonnes of carbon into the atmosphere, bringing the cumulative fossil fuel total up to 965 gigatonnes. By controlling methane you have indeed kept the warming in 2040 from broaching the 2C limit, but what happens then? In order to keep the cumulative emissions below the 1 trillion tonne limit, you are faced with the daunting task of bringing the emissions rate (which by 2040 has grown to 22 gigatonnes per year) all the way to zero almost immediately. That wasn’t very helpful, was it? At that point, you’d probably like to return the time you bought and get a refund (but sorry, no refunds on sale items). More realistically, by the time you managed to halt emissions growth and bring it down to nearly zero, another half trillion tonnes or so would have accumulated in the atmosphere, committing the Earth to a yet higher level of long-term warming.
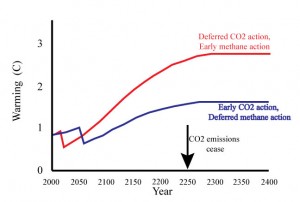
Suppose instead that you had focused all efforts on reducing the growth rate of CO2 emissions from 3% to 2%, averaged over 2010-2040, forgetting about methane until the end of that period. In this scenario, the cumulative carbon emitted up to 2040 is only 713 gigatonnes, giving more time to avoid hitting the trillion-tonne threshold. The warming from CO2 in 2040 is about 1.2C, but we have to add in another 0.4ºC because we haven’t done anything to bring down methane emissions. That brings the warming to 1.6C, which will increase further beyond 2040 as the cumulative carbon emissions approach a trillion tonnes. However, since methane responds within a decade to emissions reductions, we still get the full climate benefit of reducing methane even if the actions are deferred to 2040. The same cannot be said for deferral of action on CO2 emissions.
The following cartoon, loosely based on Eby’s calculations shows two illustrative scenarios: one in which early action is taken on methane, at the expense of allowing cumulative CO2-carbon emissions to rise to around 1.7 trillion tonnes, and another in which action on methane is delayed until 2040, allowing cumulative emissions to be held to a trillion tonnes. The curves can be diddled a bit depending on how much short term warming you get from controlling additional short-lived gases, and how much extra cumulative carbon emissions you assume goes along, but it is really hard to come up with any scenario where you come out ahead from acting early on the short-lived forcings instead of going all-out to reduce the rate of CO2 emissions.
There are a few greenhouse gases other than CO2 that have lifetimes sufficiently long to lend some urgency to their control. That would include HFC23 with a lifetime of 260 years, CFC13 with a lifetime of 640 years and SF6 with a practically unlimited lifetime. Most of the rest are more like methane than they are like CO2 (e.g HFC31 at 5 years)
Absorbing aerosols — soot, loosely speaking — have a number of complex regional effects that make it difficult to treat their climate impact on an equal footing with that of well-mixed greenhouse gases. Soot falling on snow or ice has an unambiguous warming effect, manifest particularly strongly at high latitudes and high altitudes. For airborne absorbing aerosols, though, it is hard to even know whether they have a warming or cooling effect on surface temperature, or leave it more or less unchanged. Except over high albedo surfaces, airborne aerosols mainly heat the atmosphere by direct solar absorption, at the expense of reduced solar absorption at the surface. When the shading is not too strong, the main consequence is a reduction of the convection that would ordinarily carry solar energy from the ground to the atmosphere. This profoundly influences precipitation, and the atmospheric circulation, especially in the tropics. In extreme cases, the atmospheric absorption can even shut down convection completely, leading to stabilization of the tropospheric lapse rate and a severe surface cooling, as in the Nuclear Winter limit (see also the more elementary discussion of this limit in Chapter 4 of Principles of Planetary Climate).
A further consideration is that most activities that emit soot also emit precursors to reflecting aerosols which cool the planet. It is unlikely (and probably undesirable) that one would be able to limit one without also limiting the other. Hence, the net implication of the black carbon component is probably that it will help offset some of the warming caused by eliminating sulfate aerosols. That’s good, but it’s not what you bargained for if you were expecting a cooling for your money. The main thing about soot and the stew of toxic emissions going into the Atmospheric Brown Cloud , though, is that there are compelling human health, agricultural, and regional climate reasons to eliminate them, regardless of the side effect on global temperature. These are things that need to be done regardless of the climate implications (positive or negative), just as there is a need to supply the developing world with reliable clean water. It is pointless to make an already complicated climate negotiation yet more complicated by wrapping such things into the mix. It is nonetheless worth noting that many of the things one would do to reduce soot emissions, such as substituting natural gas for coal, or burning coal in cleaner, more efficient power plants, also would tend to reduce CO2 emissions, and such double-wins are of course to be sought and pursued ardently (note Gavin’s op-ed on co-benefits of CO2 reduction).
IPCC-style Global Warming Potentials attempt to trade off radiative forcing against lifetime in a Procrustean attempt to boil all climate forcings down to a single handy-dandy number that can be used in climate treaties and national legislation. In reality, aerosol-forming emissions, short-lived greenhouse gas emissions, and CO2 emissions are separate dials, controlling very different aspects of the Earth’s climate future. CO2 emissions play a distinguished role, because they ratchet up the Earth’s thermostat. It’s a dial you can turn up, but you can’t turn it back down. CO2 is a genie you can’t put back in the bottle. Climate forcings should not be aggregated. Each category should be treated in its own right. Otherwise, there are perverse incentives to do too much too soon on short-lived forcings and too little too late on CO2.
BPL 21 we should also note that what you’re replying to is depending on partial quote mining. Nonetheless, the physics is the answer, as you said.
Did,
What part of “enough” did you not understand?
Dear darling Barton Paul:
The irony! Gavin snipped a line about your debating tactics, and here you go again.
Insults and quibbles – anything except look at the actual question under discussion.
This is perhaps the point where I should step away and ignore your sophistry – but your latest jibe passed moderation, so I thought you deserved a reply.
However, it’s the last I will say to you on the subject, until you inevitably repeat your specious claims.
Didactylos @249 — Dryland farming is dryland farming, worldwide.
The matters are relatively simple; Liebig’s Law of the Minimum writ large.
The precise dates are of course uncertain, but it does appear that all the world’s agriculture will be impacted. Even those regions estimated to have more precipitation in the future may well (therefore) become less productive. [How much yield from the Swat Valley, Pakistan, this last growing season, hmmm?]
250 David Painter,
I had a little difficulty reading your discussion, since it is hard to comprehend what the alternative is to not burning off the natural gas ‘liquids’, as I think they are called here, if they can not be transported.
But propane is not that difficult to transport, and there is a market for it, competing at a price much like that of gasoline in many cases.
On the other hand, where collection and transport of natural gas is not readily done, it is often just flared off. This is superior from a ‘green house gas’ point of view, to just releasing it into the atmosphere. But of course it puts out heat. I think the consensus is here that this is not so important as the remaining gas, either CO2 or CH4.
Well, consider the alternative:
http://eatingjellyfish.com/?tag=cassandra
re 255 Jim Bullis
The economic realitiy is that at remote gas and oil production sites perfectly usable resources are burnt off, this causes many problems due to the sole persuit of cash, transport is available but often the producer does’nt want the hasstle/costs anyway, and no-one can enforce this it seems. The environment comes way down the list of acual cash priorities of some organisations in contrast with the glossy stements.
The alternative is not to produce a byproduct if it can’t be used or stored safely by enforcement; an effect will be increased energy costs yes. The irony is most compliant oil and gas fields use this condensate to generate electricity capacity (that they can’t use anyway!) BUT electricity infrastructure is a more “hygenic” alternative to oil/gas pipelines to use this byproduct and gas generators can be “clean”.
Environmentally to dismiss the pure energy release as having no effect on the environment would be to dismiss thermals and clouds in the same way as they can both be the products of convection caused by differential air density. The volume of heated air I think is very relevant to all the discussions, as a Hot air balloon pilot I can testify that at at whatever altitude propane never completely burns off; the partially burnt gas mixture rises continually carrying the combustion products to even higher altitude until pressure and temperature stop them. unburnt propare released at altitude may not necessaryily go down (as its heavier than air) but due to convection air currents it rises, and I suspect that some of it never comes down again, certainly for kerosine at 42,000ft (aircraft).
The vast and uncontrolled volumes of flaring are producing millions of tons of lift annually, this will carry all sorts of gasses higher into the atmosphere. This relative high pressure area (as the volume expands) will have an effect on the local winds and convection patterns, and differentially more effect in colder air (the colder the environment the worse the effect).
The worlds largest hot air balloon (the energiser bunny!) has a burner capable of 30,000,000 BTU/hour and should lift the 1,170 lbs ballon plus crew to over 6000ft with ease (probably never does this though). The convection effect of flaring will affect the usual air flows and thermals defined by nominal weather patterns and geography. The large balloon 30,000,000BTU burner is tiny compared with some gas flares I have see in the north sea, the Nigerian delta has many oil flares 24hrs a day much much larger than this; but the Siberian flares I think are more harmful due to the temperature difference, local topography and the lattitude.
I don’t know enough atmospheric physics to calculate what size the effective high presssure “bubble” created over the flares would be, but it would carry small particles and gasses to higher altitudes as can be seen on any sunny day by watching thermals, the siberian flares are creating thermals in winter, in the wrong places.
To “buy time” for real understanding of the climate would be to enforce those agreements already being flouted, at those places most likely to have an adverse affect, near areas of concern.
True CO2 and CH4 are more harmful on paper, but the debates I have read seem very stuck in the mud, so to speak; in order to cause harm they have to interact with the atmosphere, in order to do that they have to get into the atmosphere above ground level. Many cars producing emissions at ground level next to the carbon sinks (ocean, trees etc) are unlikely to achieve that without convection, Flaring produces the emissions of many 1000’s of cars a day and inject this via convection directly into the atmosphere several 1000’s of feet above the reach of the traditional carbon sinks.
http://www.energizer.com/energizer-bunny/hot-hare-balloon/Pages/hot_hare_balloon.aspx
Re 257 David Painter – in your 250 comment, you gave a figure of 70,000,000 MJ/hour
In the global average, ~ 100 W/m2 is the heat flux carried away by convection. 100 W/m2 over just 1 km2 is 100 MW, or
360,000 MJ/hour; over a 20 km x 10 km area (200 km2), that’s 72,000,000 MJ/hour.
Localized heating can certainly be a local problem, and it would not surprise me if the heating can affect where some pollutants go via the heights to which they are initially carried, provided they then fall out of the atmosphere shortly. But CO2 and CH4 molecules have atmospheric residence times measured in years …
(CH4 ~ 10 years, CO2 … off the top of my head, I think it’s close to 4 years, but the molecules are ‘recycled’ back into the atmosphere from the upper ocean over a similarly short time period (from memory) and over a longer time (a couple decades?), from land surface organic C; the longevity of the perturbation of atmospheric CO2 concentration is much longer – it then goes into the deep ocean, and it reacts with dissolving carbonates, allowing farther uptake of CO2 from the air, but this still requires additional CO2 in the air; chemical weathering of carbonates also very slowly supplies carbonate ions that allows the oceans to hold more CO2; it is ultimately the chemical weathering of silicate minerals that supplies ions to the ocean that allows solid carbonate minerals to accumulate along with a net removal of CO2 from the atmosphere (this tends to balance geologic emission of CO2 over time (both from inorganic C and from organic C), except for the contribution from organic C burial)
… over which time the atmospheric circulation easily mixes the air and transports CO2 and CH4 from any one source to all over the globe. This doesn’t eliminate compositional gradients but makes them relatively small – some exceptions can be found, near the surface, near sources and sinks (under forest canopies, for example), but this affects a relatively small volume of air and has little impact on radiative forcing (generally anything under a forest canopy is not going to directly affect radiative forcing as much anyway; greenhouse gas molecules have reduced effect on the tropopause-level and TOA-level fluxes because the air near the surface tends to have temperatures similar to the surface temperature, and because they underneath greenhouse gas molecules above).
—-
Re 244 Jim Bullis – as Dappledwater explained, aragonite and calcite are both forms of CaCO3 (Pearls are made of aragonite).
but since it seems to go away with higher
CO2 concentrations that process must in itself take up CO2. Then, as the aragonite depleted water is drawn down into the
thermohaline circulation, that would seem to carry CO2 also, into the deep. Right?
Yes, the dissolution of solid carbonate mineral adds carbonate ions to the water, which can react with CO2 to form bicarbonate ions, thus allowing water to gain more CO2 from the air (if it is close enough to the surface) for the same partial pressure of CO2 (and temperature, salinity, etc.). It can then take more inorganic C (by an amount greater than the dissolved mineral content) to the deep ocean upon sinking. As I understand it, bottom water may also gain carbonate ions via dissolution of carbonate minerals and thus, upoon surfacing, take more CO2 out of the atmosphere (or release less CO2 to the atmosphere).
(this tends to balance geologic emission of CO2 over time (both from inorganic C and from organic C), except for the contribution from organic C burial)
I would just say that geologic CO2 sequestration enabled by chemical weathering of silicates, combined with organic C burial, tends to balance geologic emission of CO2 from inorganic and organic sources; however, organic C burial feedbacks don’t, so far as I know, correlate with temperature and atmospheric CO2 concentration the same way that chemical weathering tends to do (aside from enhancement of chemical weathering by glacial weathering and lower sea level during ice ages, and also, vegetation affects chemical weathering … but anyway…
245 flxble
It would seem that we understand the English language differently, and it would also seem that it might be unnecessary for you to dish out discipline based on how things seem to you.
At one time I had the impression that this site was here to help communicate the ‘climate science’ to those who are not members of the peerage. However, I attempt to understand and where things seem confusing I ask questions. I also put forward concepts that would seem to be consistent with the objectives of the scientists here. For you and others who find this annoying, perhaps you could just train your eye to skip my questions.
I much appreciate those who make an effort to communicate.
246 Dappledwater,
You seem to have some knowledge of this topic which I find confusing. I appreciate your help, though the pleasure of learning might be enhanced if we could do it without the admonishments to ‘read the peer reviewed literature’. Are you really so insular in your peer position that you do not realize how confusing adademic literature can be?
As to how we read things, it appears you are unaware of a difference between guesswork and exploratory questions. But I am not one to deliberately mislead; my questions are both pointed and exploratory, as I am not entirely convinced that the ocean effects are adequately recognized in the climate predictions. Note, I said ‘not entirely convinced’ which means I am reserving judgment.
And I also should note, I am not done. After we get the chemistry sorted out, the question of overturning of the oceans needs clarification, particularly in respect to ‘age of deep ocean water’ on which the consensus rate of overturning is based.
ccpo: I’m not disagreeing with you, but have you considered that in places like India, demand for meat is rising rapidly as a result of a growing, prosperous middle class. Economic success can lead to increased consumption, and if we look at the US or Europe, there seems to be no practical ceiling until we’re all balloon-shaped.
Supply and demand is a complicated subject, and I’m fairly confident that economists don’t understand it much either (although they hopefully have a better grasp than you or I).
Comment by Didactylos — 14 December 2010 @ 8:48 AM
To both you and Patrick: I should make clear: I don’t care about prices because I am virtually never dealing in microeconomics. They’re irrelevant (to me) wrt the long-term of where we need to end up. If it isn’t about that, it’s not on my radar. At the end of the day, prices are irrelevant wrt non-renewable and/or over-consumed resources.
Time is short and the conversation needs to change radically to dealing with the world that is coming. Once we have made some intelligent guesses as to where we need to be and some methods that can help us do that, we can start backcasting and deal with the microeconomics of getting from here to there.
I should also make clear my understanding of neo-classical/Keynesian economics is rather dismissive. I suggest Nicole Foss and Steve Keen, among others.
#
Didactylos @249 — Dryland farming is dryland farming, worldwide.
The matters are relatively simple; Liebig’s Law of the Minimum writ large.
The precise dates are of course uncertain, but it does appear that all the world’s agriculture will be impacted. Even those regions estimated to have more precipitation in the future may well (therefore) become less productive. [How much yield from the Swat Valley, Pakistan, this last growing season, hmmm?]
Comment by David B. Benson — 16 December 2010 @ 9:37 PM
I would caution against assuming what can or can’t be done in drylands, despite long experience, based on typical agricultural practices and get your hands on Mollison’s “Global Gardener: Drylands” and Geoff Lawton’s “Greening the Desert.”
re 258 Patric027
Interesting facts thank you.
The concentrated effects of a large number of concentrated heat sources scattered across a wide area of Siberian Tundra, are producing thermals and convection in an area that would not normally produce such forceful air movement, effectively an unnatural event for the “type” of local area (strong thermals over snow 24/7 365 days a year that should be below the 100W/m2 quoted)
As can be seen with many things in nature a large number of smaller processes acting together can magnify an effect, or produce something completely different. Many pilots know only a hand full of trees can produce a very forceful downdraft in windless conditions and small lakes can produce equally strong thermals from very small areas of water.
My point of concern is that just as fires “draw” upwards the air volume around them, the effect of these flares creates an effect that is very much more significant than the simple figures alone suggest; and the scale of air movement over the whole area may be causing massive air movement that is disrupting possibly the global airflow of the arctic and Kara sea.(As it interrupts direction and strength of air currents that would normally flow unimpeded here)
The location of the oil and gas fields and the apparent (by my own observations) changes in the cloud cover may suggest that these flares are reducing the formation of the “normal” clouds over this vast area, lessening the available water vapor over this area, and magnifying other solar processes(increasing forcing?). Lower clouds + lower airmass density would result with possible other consequences for deviation of fronts/storms etc.
The infra-red signatures of these flares are far in excess of the cities and towns that occupy far larger areas and are home to many 1000’s of people, so on energy efficiency grounds alone these flares are wasting resources that cannot be replaced.
An interesting article on airmass causing variations of Arctic sea Ice. “We discovered that months with very little ice cover and high temperatures corresponded with crucial variations in the wind patterns.”
http://planetsave.com/2010/04/28/russian-winds-reducing-arctic-sea-ice/
I have flown to 4000ft on approx 30Kg of propane in a 1 1/2 ton balloon (after inflation) so I think the scale of the injection of combustion products from ground level has been underestimated, considering the vast amounts of heat from these sites and their locations and their unlimited lift potential for smaller particles and molecules.
I have not seen any studies of the thermal effects of these flares.
Patrick 027
In the words of Weart, Roger Revelle discovered when he examined the chemistry of sea water that CO2 going into sea water quickly re-emerges. The chemistry is not really explained there.
Your discussion is more believable, but it would lead us to think that CO2 is being taken into the oceans very effectively, though not fast enough. And you qualify that to be at risk as we get to 450 ppm of CO2 in the atmosphere.
The key issues seem to be (1) the extent of the largest producer of calcite, being planckton, and the prospects for growth in the amount of that stuff as warming goes on, before we get to 450 ppm of course, and (2) the overturning rate of the ocean, by which CO2 held as calcium carbonate gets settled into deep water, either as the overturning circulation, or as direct falling of this heavier substance.
The deep overturning circulation has been discussed here, but it seems to be determined to be a very slow thing based on the Ewing measurements of the age of deep sea water. I am particularly puzzled about how the age of water can be measured by radio-carbon dating, since I can not imagine a long term cohesive lump of water as something that could exist. Thus, not only would precipitating substances affect the date, so would the bacteria that consume oil that leaks from the seabed in a rather general way.
If radio-carbon dating is in question, then we might look at the work of T. E. Pochapsky in measuring deep currents with neutrally buoyant floats. There are number of papers by him, some of which were under the organization of Hudson Laboratories of Columbia University, and later, Lamont Doherty organization, also of Columbia. The Pochapsky data is not a sufficient sample but it does show deep currents in the North Atlantic (East of Bermuda) that are suggestive of deep flow rates of an order of magnitude that shows fairly short overturning rates.
The point of this is that with fairly short overturning rates, the increased far Southerly winds said to be expected with a warmer globe, there would be a pick-up of the thermohaline circulation such that the dissolved CO2 as well as the bicarbonate variation you mentioned, would be better carried to deep ocean regions.
This is all a very complicated business, but I think it could be a useful discussion for the folks here, you in particular Patrick 027.
Thanks again to our hosts.
http://www.aip.org/history/climate/Revelle.htm
265 Hank Roberts
Thanks for helping with the link, here and before.
Re mine#265,
Table 2 of:
http://onlinelibrary.wiley.com/doi/10.1111/j.2153-3490.1963.tb01398.x/pdf
shows measurements from mid North Atlantic having velocities ranging from 2cm/sec to 10 cm/sec.
Jim, I guessed where you might have read Weart on Revelle. But please, cite the sources for what you claim.
I don’t read it the way you do. Others can look — if you cite your source.
You should read some of the papers that cite Revelle’s 1957 piece; you’re building on what-if-it-were-true notions. Reading the fifty years of research since Revelle wrote that bit would give you a foundation of facts.
268 Hank Roberts
Thanks.
My general point is that the oceans which have capacity to take up heat, will probably do so.
I question not at all the fact that CO2 from industrial activity would cause an accumulation of it, and that the shift of spectral position of reflected energy would lead to heat entrapment. I also see that the fact of CO2 saturation relative to the outgoing path does not mean saturation of the heat trapping effect involving successive re-radiation from different levels.
However, the point that I have intended to make is that the accumulated heat will be divided between the atmosphere and the oceans. I further suggested that the fraction going into the oceans would increase as weather activity increased, with hurricanes being one such form of activity as an example. I pointed out that this was a feedback (in the true Bode sense of feedback) which would have a moderating effect on the atmospheric temperature increases. I also cautioned that to use atmospheric temperature as the key measure of global warming could be a mistake since it only partly reflected the situation.
About a year ago the Leviticus paper was discussed here on this site, and indeed it was discovered that the upper levels of the ocean had taken up heat, and this was not something that had been anticipated in the prior modeling runs. See: Levitus et al., Geophysical Research Letters 36 (2009)
Noting that Leviticus addressed the upper levels of the ocean, I reacted based on experience going back to the 1960s in deep ocean research that the deep ocean would probably be involved more than seemed to be recognized. In conjunction with equipment installations south of Bermuda, the deep submersible Alvin had done a survey indicating some deep currents existed, though not of much concern for that activity then. I also had vague recollection of Pochapsky’s work which I knew of through conversations with Bill Branscomb who was the electronics engineer for Pochapsky. All this is to say that it simply does not seem right that the deep ocean involvement is relegated to general inactivity due to centuries long overturning rates. Though certainly sluggish in response, the Ewing carbon dating seems to have much overstated the slowness.
The above only relates to temperature. CO2 sequestration was not on my mind until recently, when I became more aware of the ocean actions in this respect. All of the overturning into the deep ocean questions become much more important, because instead of just being a mitigation of atmospheric temperature problems through real feedback, the possibility of sequestration of CO2 would remediated the real cause of the problem. And of course, this had been taken into account long ago. The Weart explanation seems to say that Roger Revelle’s view of ocean water chemistry is correct. I don’t consider it a question of chemistry since plankton is very much involved, but that is quibbling.
Weart also discusses Roger Revelle’s discovery that radioactive debris stays in layers in the ocean over 100 square kilometers. That discovery seems to be part of the basis of ocean modeling, though 100 square kilometers is a trivial area in the Pacific, and the circulation issues are far more extensive, and the real complexity is trivialized by layered ocean modeling, though the actual practice here is not clear. There seems to be confusion about a layer of water to some depth which is called a mixed layer, but is only mixed part of the year.
The paper by Pochapsky: http://onlinelibrary.wiley.com/doi/10.1111/j.2153-3490.1963.tb01398.x/pdf and others discuss his attempts to measure vertical water motion as well as horizontal motions. This is also relevant to the notion that horizontal layers are not a good generalization.
So I was interested in the Labrador Sea data showing quite a lot of warming of deep ocean water at that particular spot, which was a subject of discussion here on this website. The implications of CO2 being sequestered along with movement of warmer water did not come up, but it seems interesting, though it is clearly a complex matter which I hope would be of interest to those more competent in analysis of such things than I am. I would appreciate leads to work of that sort.
268 Hank Roberts,
I did not adequately address your last paragraph.
First, you said, “You should read some of the papers that cite Revelle’s 1957 piece – -” Ok. This seemed to be a fruitless endeavor; any suggestions of papers that would really clarify things?
Second, you said, ” – – you’re building on what-if-it-were-true notions.” Ok, what do you think I am building? I am not in position as an outsider to build much of anything. I look to relate things I know about to the reports from climate science, as I hope I showed in my previous.
Third, you said, “Reading the fifty years of research since Revelle wrote that bit would give you a foundation of facts.” I suppose so. However, I do not intend to do such a thing; rather, I intend to ask selected questions of those who are familiar with that research; hopefully I will be able to read incisively regarding specific issues.
In some of my past careers I have made myself useful by questioning of well acknowledged authorities. I am interested in the answers that might come out of this; of course, nobody is obligated to do anything, but I try to make my questions important so that others would be interested.
Re 264 Jim Bullis –
I never stated that oceanic sequestration of CO2 was at risk specifically at 450 ppm, though I am aware that climate itself can affect this process (hence large ice age CO2 feedback, of course); if someone else mentioned 450 ppm, perhaps there was a good reason for it. I wasn’t trying to imply any level of effectiveness in oceanic uptake of CO2; I’m not familiar with a lot of the numbers in this area, just the conceptual/qualitative explanations.
Formation of solid carbonate minerals will reduce carbonate ions, causing a net reaction of bicarbonate ions to carbonates and CO2. Thus CaCO3 forming and then falling from surface waters would reduce the upper ocean’s ability to hold CO2 (opposite effect of dissolving CaCO3). Addition of Ca and carbonate ions from chemical weathering of silicate minerals, however (where said carbonate ions came from CO2) can supply ions to allow CaCO3 formation while maintaining composition and ability to hold CO2.
My understanding is that the residence time of a water parcel in the deep ocean (below the upper mixed layer) is ~ 1000 years. This is an average time; some water masses that sink will surface in a shorter time or longer time. Water masses, like air mass (actually perhaps much better than air masses (lack of heating and cooling, evaporation and precipitation in the deep), for potential temperature and density), can retain some characteristics and be identified by source regions (Antarctic bottom water). They eventually could mix (and presumably either mix with surface water as a way to get back to the surface, and/or else they upwell as distinct water masses and then eventually gain characteristics of surface water); this takes a lot longer than air masses because the oceanic circulations are much slower.
… of course, as long as some small CaCO3-shelled organisms are floating around, they can supply the ocean with more dissolved CaCO3, helping the ocean to take up more CO2; presumably coral reefs will take longer to dissolve and will remain after all the floating shells have gone (surface area/volume), so at such a point the supply of CaCO3(aq) may be smaller, although the decreasing pH of rain itself (from CO2, setting aside sulfuric and nitric acids or reduction of those) may enable greater supply of CaCO3(aq) from land (weathering of carbonates – maybe silicates too), depending…
This being before climate feedbacks are taken into account.
But we don’t really want to have to dissolve everything (and presumably this would affect the organic C cycling, and sea food, etc. PS some nutrients are returned to land upon bears eating salmon!). (Better to sacrifice ‘dead’ CaCO3 than that currently in use; I suppose we could start grinding up the limestone – would that be easier than peridotite?)
A = 1.386e+18 ocean volume m3 ga.water.usgs.gov/edu/watercycleoceans.html
B = 5.67648e+14 AMOC m^3/year http://www.geosc.psu.edu/~kzk10/baehr_etal_cc_07.pdf [1]
A/B = 2441.6539827 years
Or about 1200 years to swap the bottom half with the top half. (Ignore the false accuracy from cut’n paste from Appleworks)
The bottom & top half don’t just get swapped. Upwelling off Portugal, and the Benguela upwelling return some deep water to the surface, which gets carried by the Azores & Benguela currents to the Atlantic Equatorial current, and returned to the Gulfstream in much shorter time frames – http://oceancurrents.rsmas.miami.edu/atlantic/atlantic-arrows.html.
[1] http://www.ipcc.ch/publications_and_data/ar4/wg1/en/ch10s10-3-4.html
“Observationally based estimates of late-20th century MOC are shown as vertical bars on the left” (which range from ~12 to 22 Sv; I used 18)
“The MOC is not necessarily a comprehensive indicator of ocean circulation changes in response to global warming.”
271 Patrick 027
Right you are about having not said 450 ppm. Sorry.
That was #246 Dapplewater.
I am specifically challenging the notion that water is resident anywhere for 1000 years, let alone all water below the upper mixed layer, which is not all that deep. That might have been what Revelle thought based on his 100 square kilometer sample area.
By the way, the upper mixed layer, when it exists at all, can be quite shallow. There seems to be some confusion about what the upper mixed layer really is, as I previously noted. If it is defined as a fixed geometrical line, then it would be the depth of the mixed layer as it appears seasonally. During the rest of the year when a thermocline dominates, the layer boundary would have to be only as a conceptual definition.
By the way, your idea of neutralizing ocean acidity is interesting. That might buy time if the 450 ppm number from Dapplewater is real.
Re 274 Jim Bullis – the layer above the thermocline of course varies in thickness and dissappears in some places. I wonder about the seasonality – what about lower latitudes? Of course the smaller temperature variations still allows wind changes, precipitation/evaporation changes, etc.
But I think conceptually it is the layer of water that can come to thermal and compositional equilibrium with the atmosphere above over some time period, perhaps a year or so? Perhaps it could be considered an equivalent volume – the fuzzy edges being divided between the mixed and deeper layer (?).
More rapid exchange with deep water would tend to delay the approach to climatic equilibrium initially (but reduce the longevity of a remaining residual disequilibrium) but by itself the greater rate of heat capacity exchange would not change the equilibrium (for a given CO2 amount). It would affect CO2, but so far whatever effect it is having, we’ve seen it.
… differentiating between a mixed layer (and thermocline) that exists at any one time and a climatic mixed layer (and thermocline) – or perhaps we should just say the upper ocean (?)
But I still have the impression that at any instant, over much of the globe, a mixed layer can be found.
J Bullis @ 274 – By the way, your idea of neutralizing ocean acidity is interesting.
Except of course, it isn’t his idea. CaCO3 compensation might be novel to you, however research on this topic is decades old . It’s even discussed on a previous post at Real Climate:
The Acid Ocean – the Other Problem with CO2 Emission
“The natural pH of the ocean is determined by a need to balance the deposition and burial of CaCO3 on the sea floor against the influx of Ca2+ and CO32- into the ocean from dissolving rocks on land, called weathering. These processes stabilize the pH of the ocean, by a mechanism called CaCO3 compensation. CaCO3 compensation works on time scales of thousands of years or so.
Yes, it operates on millennial timescales, not much use when the Arctic Ocean will likely be corrosive to marine organisms within a decade, and the Southern Ocean by 2030.
If you’re willing to learn, here’s a paper that gives you an idea, why the experts are concerned, and has a basic run down on ocean chemistry:
Ocean Acidification in Deep Time
Here’s the abstract:
“Is there precedence in Earth history for the rapid release of carbon dioxide (CO2) by fossil fuel burning and its environmental consequences? Proxy evidence indicates that atmospheric CO2 concentrations were higher during long warm intervals in the geologic past, and that these conditions did not prevent the precipitation and accumulation of calcium carbonate (CaCO3) as limestone; accumulation of alkalinity brought to the ocean by rivers kept surface waters supersaturated. But these were steady states, not perturbations.
More rapid additions of carbon dioxide during extreme events in Earth history, including the end-Permian mass extinction (251 million years ago) and the Paleocene-Eocene Termal Maximum (PETM, 56 million years ago) may have driven surface waters to undersaturation, although the evidence supporting this assertion is weak.
Nevertheless, observations and modeling clearly show that during the PETM the deep ocean, at least, became highly corrosive to CaCO3. These same models applied to modern fossil fuel release project a substantial decline in surface water saturation state in the next century. So, there may be no precedent in Earth history for the type
of disruption we might expect from the phenomenally rapid rate of carbon addition associated with fossil fuel burning.”
JB 274: I am specifically challenging the notion that water is resident anywhere for 1000 years
BPL: Crack an oceanography textbook. It’s not controversial.
278 BPL
I have no problem with finding fault with things that are not controversial, if they disagree with facts.
Velocities in the 2 to 10 cm/sec are not consistent with 1000 year stability. This is from one of several papers by Pochapsky from the 1960s.
See: http://onlinelibrary.wiley.com/doi/10.1111/j.2153-3490.1963.tb01398.x/pdf
Do you have an oceanography textbook that discusses the radio-carbon dating by Ewing, on which the 1000 year kind of numbers seem to be based, according the Weart (http://www.aip.org/history/climate/Revelle.htm — from Hank Roberts link)
I would look specifically for discussion of how dating could be valid for something continuously in presence of new sources of carbon, the new sources being oil seepage from the ocean floor that are consumed by bacteria. I also would look further at the possibility that ancient calcium carbonate materials would also have released some CO2 along the way. Hence, the Ewing notion that CO2 in his water samples had to have come from atmospheric CO2 seems inappropriate. And on that basis the radio-carbon dating should be thrown out.
Thus, we are left to look at actual data showing deep ocean water motions, as in the referenced Pochapsky work.
277 Dappledwater
Thanks for your link to a paper by which I could learn, though it was disappointing.
I am not very willing to learn how things will be when all the fossil fuel of the world is exhausted, though it is interesting that even this uninteresting extremity of conditions will reduce ocean pH by only .7 . And that all fossil fuel use in previous history has reduced pH by .1. I wondered as I read if the authors realize how much coal we have. The studies of that resource indicate the limit is only based on how much dirt we are willing to scrape off the enormous coal basins. I accept that things can not be carried to that extreme. However, it is more useful to think about stretching out the time over which coal is used, and this is not an unreasonable way to find balance.
David B. Benson:
I didn’t notice your comment there. Yes, water is a limiting factor. And, despite talk of plants possibly needing less water in CO2-rich environments, I think we can regard it as an absolute limit, practically speaking.
So, let’s get practical. I’m not a farmer, and nor are you (but we can pretend for a moment). There isn’t just one crop. There are many choices, and many varieties of each. A good farmer will choose crops suited to his climate and other conditions.
Let’s face it: there are crops that will grow in much more hostile, hotter, dryer conditions than typically found in the continental United States. Probably not as nutritious as some other crops (but scientists are already working on that). Maybe not a good cash crop in the current economy – but that will change as the world changes. When you talk about a farmer’s crop failing, that means that he chose badly with respect to the weather that year. Unusual years are going to wipe anyone out. Lots of unusual years? Time to change old habits.
Liebig’s law doesn’t mean we have to trip over and fall flat at the first step. With modern sustainable farming, it’s a much longer road to a dustbowl.
Flooding? Well, that comes down to water management again, doesn’t it. If you’ve got it, you’ve got full reservoirs, water for the dry season and protection against erosion. If you don’t, you’ve got no crop, no home, and no fertile soil left.
Water management. I’m beginning to think it is the only thing that will keep most of us alive while the world wakes up and takes the action we should already be taking.
Didactylos says: “Let’s face it: there are crops that will grow in much more hostile, hotter, dryer conditions than typically found in the continental United States.”
Yup, and they typically yield far fewer calories, grams of protein, nutrition… than do the crops we grow now. If you look at Guns, Germs and Steel, you see that the crops that are grown in a region are grown there for a reason. True, genetic engineering may help, but I don’t think it can keep up with need. It is not simply a matter of moving the US wheat crop to Canada. The Canadian Shield isn’t a very fertile palce. Keep in mind that if we only had a billion people on Earth, we might be able to negotiate the challenges that face us. If we had unlimited sources of clean energy, we could probably get by. It is the combined challenges of overpopulation, environmental degradation, resource depletion, fertility depletion, fisheries collapse, the end of Fossil fuels, etc. And then if we manage to negotiate these obstacles and develop a sustainable economy, we have to figure out how to make it work as population returns to sustainable levels.
281 Didacto
Water management, you don’t say. Could that concept extend to a long aquaduct?
ccpo @262 — Of course the farmers around here are fairly knowledagable and receive advice from the state ag extension agents. Despite all of that, right here it remains usually 2 years of soft white winter wheat and then a year of dry peas or lentils. Of course small amounts of other corns are grown as well.
But only 100 km to the west, at lower elevation, those unable to irrigate have to leave the fields fallow every other year to accumulate enough ground moisture.
So thanks for the references, but water is an absolute requirement.
Ray Ladbury @282 — Yup.
Want to run for president?
Re 281 Didactylos A good farmer will choose crops suited to his climate and other conditions.
How good are most of the farmers we have? Not that it’s their fault for not adapting sometimes. There are issues with U.S. agricultural policy that have to be cleared up. And ‘big ag’. Education. Variety in food (consumers will also have to adapt. PS not everyone needs to adapt by eating different foods; the price signals will ‘direct’ those who are willing and able to try something else to the alternatives. I mention this because I can imagine some people with allergies and metabolic conditions may be concerned about the idea that ‘we all’ have to ‘do our part’ – well maybe we do but that doesn’t mean it’s the same part for everybody. For that matter, people can still differentiate according to personal preferences, as they do now (I’m being optimistic about lack of famine)). Ideally, a farmer will be able to change plans when planting must be delayed, to a crop with a shorter season, or a crop that can be planted in those conditions (buckwheat for example – I think if the ground is too wet for wheat, as I recall). Failed/spoiled crops and food waste should still be useful – feed, biofuel, chemical feedstocks…
Solar power plants in marginal lands could be used to focus precipitation into smaller areas (bordering power plants), essentially producing a locally wetter climate.
Ray Ladbury wrote in 282:
I haven’t read the book, but, but you have gotten me interested. However, to amplify a little…
We know thatstronger equatorial moist air convection is causing the Hadley Cells to expand, but at roughly three times the rate that we expected. In the Hadley Cells the near-equator is where moist air rises, giving up moisture in the form of precipitation as it expands and cools with increased altitude.
As the air cools and dries out it falls (“subsides”) on the far from equator side of the Hadley Cell, resulting in a dry region, the high pressure, subtropical “horse latitudes,” the deserts of Africa, the Middle East and Northern Mexico. Expansion of the Hadley Cells pushes the region where air circulation brings drier, high altitude air down (where the air “subsides”) after having lost it moisture due to precipitation as it rose nearer the equator further from the equator, drying out the mid-latitudes.
Likewise, ocean warms more slowly than land due to ocean water’s greater thermal inertia. This implies that as moist ocean air is blown inland, while its absolute humidity may remain the same as when it was over the ocean its relative humidity will drop — leading to less precipitation in the interior continent. Combined with a higher rate of evaporation due to higher temperatures (with the rate of evaporation doubling for every 10°C) the interior will tend to dry out, and as the interior dries out this will lead to a reduction in inland moist air convection that will tend to increase daytime temperatures further.
The high latitudes of Canada may take over for the reduced harvests of the desiccated US to some extent, but so much of the soil in Northern Canada — where farming isn’t already taking place — has been locked in ice, will subside with the melting and draining of thermokarst lakes. Rocks will have to be cleared. And the shield itself consists of thin soil covering rock.
High latitude soil will provide a poor substitute for what had once existed in the midlatitudes. Tundra is acidic and brown forest soil becomes acidic with increased precipitation. And of course the infrastructure to support farming will be largely missing. But with temperature continuing to rise more quickly in the high latitudes than the rest of the globe it won’t make much sense to invest heavily in farming. Whatever crops the land might support will tend to be gone a few decades later.
Meanwhile to the South the coasts will provide some solace from the baking of the continental interiors but cities will keep having to be moved inland — and we will no longer be able to afford the high investment in infrastructure. Even once the temperatures plateau the oceans will continue to rise with the melting of the ice, the loss of glaciers and the icesheets that reside on land, and most importantly the expansion of the oceans.
Much of our ocean harvests come from the coral reefs which act like tropical rainforests in preserving diversity in an otherwise largely desolate ocean. But coral reefs will largely disappear due to periods of high temperature and increased acidity. And the latter of these will eat away at the calcareous protists that lie at the very base of so much of the ocean food chain.
Increased drought and the occasional flooding will put at risk much of the fresh water supply — already threatened by our depletion of the water tables and many of our freshwater lakes. What water flows will tend to evaporate more quickly — with the rate of evaporation doubling for every 10°C. Rising ocean levels will likewise result in the contamination of water tables by salt and even red algae. Stronger storms will likewise contribute to this.
With shortages in freshwater you will see people increasingly relying on whatever water they can find — despite the risks. And the risks will include waterborne illnesses. The places that are hardest hit will become a breeding ground for disease. Likewise the resource shortages will make people desperate, vulnerable to extremist ideologies — and with war comes the spread of further disease.
Re 287 Timothy Chase, In the scenario presented in “Earth 2100”, it was a plague (brought on in part by climate-related poverty) that was the death blow to modern global civilization, essentially causing a famine by reducing trade – causing global population to plummet, ultimately disintegrating the U.S. into pieces (too many unsolvable disasters and people lose faith in their governments); the subsequent war between India and China wasn’t described in detail.
PS of all disaster movies I’ve seen, that was the scariest/most depressing, because it was, so far as I can tell, the most realistic (Discovery Channel’s “Supervolcano” also a bit scary, was somewhat realistic; “Day After Tomorrow” and “Armageddon” had realistic aspects but also some rather unphysical or unlikely stuff; “The Core” was totally unrealistic – a few simple facts were nominally correct, everything that happened was just wrong. But I enjoyed watching it. (insert your Fox News joke here))
JB 279: I would look specifically for discussion of how dating could be valid for something continuously in presence of new sources of carbon, the new sources being oil seepage from the ocean floor that are consumed by bacteria. I also would look further at the possibility that ancient calcium carbonate materials would also have released some CO2 along the way.
BPL: I would not expect people who deal with the ocean professionally to have missed obvious problems for decades.
Ray Ladbury: I did note the nutrition aspect. Just think! The west will be benefiting from research done (and still being done) to aid developing countries. It’s not just GM crops. There are lots of researchers using traditional cross-breeding informed by genetic analysis and understanding the role of specific proteins. What’s more, creating varieties that aren’t “owned” by massive corporations – but a discussion of the idiocy that is US patent law is really, really off-topic.
Obviously, none of the mitigating and adapting methods I’m discussing will work forever. They all have limits. But to me, the idea that humanity won’t try to adapt no matter what happens – that’s just not reasonable.
Jim Bullis: Only if there is unlimited money available and no better solutions to be found.
Patrick 027: I’m sure mistakes will be made on the way. But these days, I imagine most farmers are better informed than in past generations. More science, less folk wisdom. That’s a recipe for success when the assumptions of the folk wisdom break down.
Tim Chase: Your comments seem to be yet another nail in the coffin for monocultures. So why are they still grown? Eggs, basket etc. And that’s without touching on biodiversity.
Patrick 027 wrote in 288:
Well, obviously I could have included a bit more, but I didn’t want to paint too bleak a picture.
However there is some good news. As the occasional flash floods supercharged by the acceleration of the water cycle carry away the once rich but now dessicated topsoil they will expose then wear away rock. This leads to an accelerated rate at which atmospheric carbon dioxide is mineralized and both it and the acidity levels of the oceans are brought down.
One of the most important negative feedbacks. Unfortunately this wearing away of rock and consequent mineralization of CO2 tends to act on the scale of tens of thousands of years. The carrying away of topsoil is no doubt much faster.
Ray Ladbury wrote: “… they typically yield far fewer calories, grams of protein, nutrition… than do the crops we grow now …”
Most of the nutritional value of “the crops we grow now” in the USA is squandered by feeding those crops to cows, pigs, chickens and other animals (e.g. 98 percent of the US soybean crop is used for livestock feed) to mass-produce cheap meat, with a resulting LOSS of protein available for human consumption of up to 90 percent.
J Bullis @ 280 – it is interesting that even this uninteresting extremity of conditions will reduce ocean pH by only .7
We can add that to the long list of things about ocean acidification you don’t understand. Small changes on the pH scale represent large changes in hydrogen ion concentration. pH is an inverse logarithmic scale. Each whole unit on the scale represents a ten-fold change in hydrogen ion concentration. E.g. a pH of 7 has ten times as many hydrogen ions as a pH of 8.
A reduction in the ocean pH of 0.7 represents an over 400% increase in hydrogen ions from todays pH. Along with the other warming induced changes in the ocean(e.g. stratification & reduced nutrient upwelling), that would pretty much be curtains for almost all life in the ocean.
And that all fossil fuel use in previous history has reduced pH by .1
Yes, a roughly 30% increase in hydrogen ions from pre-industrial times. Perhaps why so many calcifying marine organisms, such as coral, are exhibiting decreased rates of calcification.
And business-as-usual scenarios project a further 0.3 – 0.4 unit lowering of ocean pH by the end of this century. A 100- 150% increase in hydrogen ions over today.
Yes, there’s a good reason why oceanographers are concerned about the rapid onset of ocean acidification, that reason being understanding.
Didactylos, Who is saying humanity won’t TRY to adapt? However, just what conditions should we expect. How do you “adapt” to a situation when rainy seasons are not predictable–when one year you have a severe drought and the next year your seed or crops are washed away by flood before you can harvest. Agriculture has only existed during a period of exceptionally stable climate. Do you think that humans only had the ability to domesticate plants as of 10000 years ago? Or perhaps did a settled agricultural lifestyle suddenly increase the number of calories we could harvest over that of a hunter-gatherer mode of existence only when the climate became relatively stable?
“Archaeologists have unearthed the remains of over 20 successive settlements in Jericho, the first of which dates back to 11,000 years ago.” from
http://en.wikipedia.org/wiki/Jericho
“More stable living patterns gave rise by around 14,000 BCE [~16,000 years ago] to a Mesolithic or, as some scholars argue, Neolithic culture, but with some characteristics of both.” from
http://en.wikipedia.org/wiki/J%C5%8Dmon_period
Both societies are usually considered to be proto-agricultural, that is with seed selection but without the cultivation typical of traditional agricultural practice. At least the Jomon peoples started these settled ways around the time of LGM, seawards of the glaciers on Hokkaido and Honshu.
293 Dabblewater
Thanks for helping us understand your superiority in oceanography, by virtue of your announcement of my lowly position on the scale; correct though that might be.
It is always interesting to calibrate the superior people though. We now know that ‘acidification’ that gets us half way to an acidic condition is a hundred years away, and that the ‘curtains’ for humanity will occur, in another 100 years or so, and that is a ‘rapid’ development.
This is not the issue of course. The issue is whether lessened alkalinity will be harmful to sea life. I would be interested in what superior folks would think about where the pH point would be that plankton stopped growing, and then what would the pH point be where calcite or aragonite would begin dissolving due to dissolved CO2.
289 BPL
It took a hundred years for Newton’s pebble theory of light to be thrown out, even though the fact of diffraction had been established much sooner.
So let’s make this all about defects of character rather than about interesting data. That’s the ticket to progress.
#287–
Timothy, I grew up in Canadian shield country. A lot places, if you “clear the rocks,” what you have left is bedrock. Sure, there are pockets of arable soil here and there, at places where sedimentation has been relatively high for long enough. But by and large, northern Canada–if it takes up more of the burden of agricultural production–will be doing it via either grazing or hydroponics, IMO. And it will be because food prices are way the heck up from what we are accustomed to now.
J. Bullis – We now know that ‘acidification’ that gets us half way to an acidic condition is a hundred years away,…………….. and that is a ‘rapid’ development
Errrrr, No. Remember the previous links?. Arctic Ocean corrosive to aragonite within a decade. Other studies reveal the Southern Ocean will be corrosive to aragonite by 2030. The Great Barrier Reef essentially dying within the same timeframe (acidification & bleaching from too warm oceans).
Remember the highlighted portion from Kump 2009?. The current rate of acidification is likely unprecedented.
This is not the issue of course.
Maybe not to you (yet), but very important to marine life.
The issue is whether lessened alkalinity will be harmful to sea life.
Kind of the whole point of the links provided. For example, 4 of the 5 previous major extinction events lead to the complete loss of corals from the world’s oceans. It took many millions of years for new forms to evolve. Those were rates of acidification much slower than today. Current observations reveal worrying trends for life in the oceans. Being dead is an impediment to adaptation, hence the scientific community’s concern.
and then what would the pH point be where calcite or aragonite would begin dissolving due to dissolved CO2.
See link provided @ 246.
“… calcifying organisms are a special case as carbonate minerals will be less saturated—and for the case of aragonite, undersaturated in surface waters in a high-CO2 ocean…. the rest of the microbial community should not be assumed to be at risk ….”
http://www.nature.com/ismej/journal/v5/n1/full/ismej201079a.html
http://cmore.soest.hawaii.edu/oceanacidification/