Are the heat waves really getting more extreme? This question popped up after the summer of 2003 in Europe, and yet again after this hot Russian summer. The European Centre for Medium-range Weather Forecasts (ECMWF), which normally doesn’t make much noise about climate issues, has since made a statement about July global mean temperature being record warm:
Consistent with widespread media reports of extreme heat and adverse impacts in various places, the latest results from ERA-Interim indicate that the average temperature over land areas of the extratropical northern hemisphere reached a new high in July 2010. May and June 2010 were also unusually warm.
Here, the ERA-Interim, also referred to as ‘ERAINT’, is the ECMWF’s state-of-the-art reanalysis. But the ERAINT describes the atmospheric state only since 1989, and in isolation, it is not the ideal data set for making inferences about long-term climate change because it doesn’t go all that far back in time. However, the statement also draws on the longer reanalysis known as the ERA40 re-analysis, spanning the time interval 1957-2002. Thus, taken into context of ERA40, the ECMWF has some legitimacy behind their statement.
The ERAINT reanalysis is a product of all suitable measurements fed into a model of the atmosphere, describing all the known relevant physical laws and processes. Basically, reanalyses represent the most complete and accurate picture that we can give for the day-to-day atmosphere, incorporating all useful information we have (satellites, ground observations, ships, buoys, aircrafts, radiosondes, rawinsondes). They can also be used to reconstruct things at finer spatial and temporal scales than is possible using met station data, based on physical rules provided by weather models.
The reanalyses are closely tied to the measurements at most locations where observations – such as 2-meter temperature, T(2m), or surface pressure – are provided and used in the data assimilation. Data assimilation is a way of making the model follow the observations as closely as possible at the locations where they are provided, hence constraining the atmospheric model. The constraining of the atmospheric model affect the predictions where there are no observations because most of the weather elements – except for precipitation – do not change abruptly over short distance (mathematically, we say that they are described by ‘spatially smooth and slowly changing functions’).
There are also locations – notably the in the Polar regions and over Africa – where ground-based measurements are sparse, and where much is left for the weather models to predict without observational constraints. In such regions, the description may be biased by model shortcomings, and different reanalysis may provide a different regional picture of the surface conditions. Surface variables such as T(2m) are strongly affected by their environment, which may be represented differently in different weather models (e.g. different spatial resolution implies different altitudes) and therefore is a reason for differences between reanalyses.
Furthermore, soil moisture may affect T(2m), linking temperature to precipitation. The energy flow (heat fluxes) between the ground/lakes/sea and the atmosphere may also affect surface temperatures. However, both precipitation and heat fluxes are computed by the reanalysis atmosphere model without direct constraints, and are therefore only loosely tied to the observations fed into the models. Furthermore, both heat fluxes and precipitation can vary substantially over short distances, and are often not smooth spatial functions.
While the evidence suggesting more extremely high temperatures are mounting over time, the number of resources offering data is also growing. Some of these involve satellite borne remote sensing instruments, but many data sets do not incorporate such data.
 In the book “A Vast Machine“, Paul N. Edwards discusses various types of data and how all data involve some type of modelling, even barometers and thermometers. It also provides an account on the observational network, models, and the knowledge we have derived from these. Myles Allen has written a review of this book in Nature, and I have reviewed it for Physics World (subscription required for the latter).
In the book “A Vast Machine“, Paul N. Edwards discusses various types of data and how all data involve some type of modelling, even barometers and thermometers. It also provides an account on the observational network, models, and the knowledge we have derived from these. Myles Allen has written a review of this book in Nature, and I have reviewed it for Physics World (subscription required for the latter).
All data need to be screened though a quality control, to eliminate misreadings, instrument failure, or other types of errors. A typical screening criterion is to check whether e.g. the temperature estimated by satellite remote sensing is unrealistically high, but sometimes such screening may also throw out valid data, such as was the case of the Antarctic ozone hole. Such post-processing is done differently in analyses, satellite measurements, and reanalyses.
The global mean temperature estimated from the ERAINT, however, is not very different from other analyses or reanalyses (see figure below) for the time they overlap. We also see a good agreement between the ERA40 reanalysis, the NCEP/NCAR reanalysis, and the traditional datasets – analyses – of gridded temperature (GISTEMP, HadCRUT3v, NCDC).
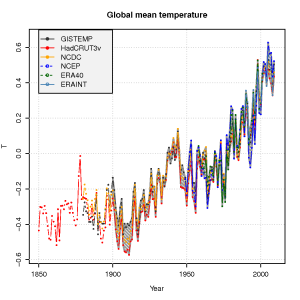
Do the ERAINT and ERA40 provide a sufficient basis for making meaningful
inferences about extreme temperatures and unprecedented heat waves? An important point with reanalyses, is that the model used doesn’t change over the time spanned by the analysis, but reanalyses are generally used with caution for climate change studies because the number and type of observations being fed into the computer model changes over time. Changes in the number of observations and instruments is also an issue affecting the more traditional analyses.
Since the ERAINT only goes as far back as 1989, it involves many modern satellite-borne remote sensing measurements, and it is believed that there are less problems with observational network discontinuity after this date than in the earlier days. It may be more problematic studying trends in the ERA40 data, due to huge improvements in the observational platforms between 1958 and now. Hence, it is important also to look at individual long-term series of high quality. These series have to be ‘homogeneous’, meaning that they need to reflect the local climate variable consistently through its span, not being affected by changes in the local environment, instrumentation, and measurement practices.
An analysis I published in 2004, looking at how often record-high monthly temperatures recur, indicated that record-breaking monthly mean temperature have been more frequent that they would have been if the climate were not getting hotter. This analysis supports the ECMWF statement, and was based on a few high-quality temperature series scattered across our planet, chosen to be sufficiently far from each other to minimize mutual dependencies that can bias the analysis.
The ECMWF provides data for some climate indices, such as the global mean temperature, and the National Oceanic and Atmospheric Administration (NOAA) has a web site for extreme temperatures and precipitation around the world with an interactive map, showing the warmest and coldest sites on the continents. Another useful tool is the KNMI ClimateExplorer, where people can both access data and carry out different analyses on line. It is also possible to get climate data on your iPhone/iPod Touch through Apps like Climate Mobile.
Update: I just learned that NOAA recently has launched a Climate Services Portal on www.climate.gov.
Update: http://rimfrost.no/ is another site that provides station-based climate data. The site shows linear trends estimated for the last 50 years.
JB 442: When advocates talk without distinguishing the difference, one should become suspicious of their motives or good sense.
BPL: But are you not yourself an “advocate” for the cogeneration machinery you are pushing? Which require fossil fuels? And would that not tie in with your beliefs that A) renewables can never replace fossil fuels completely, B) any taxes on CO2 emissions would ruin the economy, and C) windmills are useless?
JM 447: We will not, however, eliminate their use or get to near zero C02 emissions in the next 100 years. The good thing is we do not need to.
BPL: Yes we will and yes we do.
I’m afraid I agree with Didactylos. The approach we need to take is to dedicate ourselves to finding solutions. It is pointless to wring our hands about birds dying due to windmills. Windmills are going to be built, and siting will not always be ideal. Could we keep birds away from the area by broadcasting the calls of birds of prey?
Likewise, it is pointless to speculate whether peak oil is now or in 60 years, because we can’t afford another 60 years of fossil fuels, AND we can’t afford to run out of oil in another 100 years as it will likely remain essential to feeding people and providing feedstock for chemicals and carbon-based materials. Oil is simply too valuable to burn.
The ultimate problem is that within a few decades, global population will peak (we hope) at about 10 billion people. This will happen just as the effects of climate change really start to be felt and as we start to severely damage the productive capacty of the planet. Somehow we must feed all those people and keep them out of misery without irreparably damaging the global ecosystem. We must then adjust our economy to cope with the demands of a decreasing population, inverted age pyramid, resource depletion, etc. Finally, we must develop a sustainable economy, including a sustainable energy infrastructure, that remains dynamic and vibrant.
In short, we must not only utterly change te way humans live, we must do so while negotiatin a population, economic, resource, ecological and climate crisis that poses an unprecedented threat to human civilization. We have to negotiate that crisis while keeping the Earth a place where future generations can and would want to live. That, ladies and gentlemen, is the only relevant work of our generation and the next. If we fail, then everything humans have done is irrelvant.
Mike Flynn @ 450.
I rather think that your depiction of the heating and back radiation problem overlooks the basic problem. Solar irradiation never, ever stops.
Your scenario might look OK for a particular spot on the surface which is only heated for half a day. But the earth is a sphere and _half_ of it is being heated by the sun all the time, and _all_ of it is receiving back radiation all the time. One is due to the earth’s rotation, the other to the well-mixed atmosphere.
The fact that these factors vary a bit from place to place or time to time doesn’t change the overall picture. The earth as a whole never gets the chance to cool in the way you suggest.
Mike Flynn writes:
> calculate the surface temperature of the Earth
> assuming no atmosphere, and solar insolation.
Try doing this as a thought experiment:
assume the moon: No atmosphere; same distance from the Sun
I can’t quite tell what you mean when you write
> If I am correct, [the surface temperature of the Earth]
> should be a lot higher that what is actually observed.
Estimates vary. Artemis makes a valiant effort
http://www.asi.org/adb/02/05/01/surface-temperature.html
As a picture: http://www.asi.org/adb/02/05/01/surface-temp-chart.html
So that agrees with Spencer Weart’s book.
You’ve read Weart? First link under Science in the sidebar here.
Mike Flynn(450): Incoming solar radiation heats the surface in the first instance. OLR radiation cools the surface as does convection and evaporation. Back radiation (that atmospheric radiation that goes to the surface) then heats the surface. The back radiation comes from a return of some of the original OLR, re-radiation of some of the solar radiation that got absorbed in the atmosphere, and a little inherent radiation. This back radiation is in part dependent of the “initial” surface temperature and OLR, but it is not limited by it.
“… less than a meter beneath the surface of Luna will experience a very constant temperature equal to its mean surface temperature. That’s about -9°F (-23°C).” from the link I gave above
My parents own a 22-acre forest in South Dakota. I just walked the property. There are dead birds – mostly robins, blackbirds, and pigeons – all over the place. There are no windmills at all. My sister speculates that the birds, which appear to be perfectly good birds that seemingly have just dropped dead in flight, are being killed by a fast acting virus – maybe West Nile.
Now, if there was a handy windmill, perhaps we could blame it.
Ray # 454 you word things quite differently and while you and I are not in 100% agreement, I agree far more with you.Oil is too precious to use up and windmills need to be built. Since solar panels int he desert will get damaged by wind gusts of dirt and debris and the high temps make them less efficient that is not a complete option. Since windmills are horribly inefficient they need back up generation and for hydroelectric to work you need the water supply based upon location for that to work. I am not just concerned about burds and infrasound with windmill siting but also with getting maximum energy conversion and staying away from compost piles as New Scientist reported on.
All in all I am hopeful but these technologies are not going to phase out all fossil fuel burning. Overpopulation is a huge issue Ray, I definitely see the need for clean alternative energy sources to be sure.
I am hopeful too for future organic solar panels even with their current energy inefficiencies. Silcon based inorganic panels are not nearly viable but some bugs in the organic based ones makes them about 10 years off from mass production.
BPL: we cannot because we do not have the technology to store and transfer the energy needs of the world. The geopolitics, socio-economics and the fossil fuel lobby are other reasons. You need around 1500 MW of power supply from windmills to power around 400,000 homes. A 7 MW turbine is huge but we really need like 10-12 MW turbines and lots of them plus a lot of hydroelectric and solar. Even then the rising global needs are not going to be met with such large projections for future population growth.
We need to balance renewables with carbon capture, re-forestation, cleaner burning fossil fuels, and cutting back on new population growth.
JCH the bird issue was more as an aside and one of not small sarcasm. Since the serious issues of the windmills, solar panels, (inorganic and organic based) hydroelectric and such were largely ignored I brought up the birds:)
To my surprise no one has brought up the Weibull model, Betz law and the drawbacks of back up generation for these wind turbines.Well Betz was hinted at by disucssion of back up generation.
The links I provided show why we cannot power the whole world onn renewables alone. The links provided for me do not solve all of the problems they claim to.
> West Nile?
Surveillance page — worth checking, by state and by county.
It’s a serious concern, worth reporting.
http://www.cdc.gov/ncidod/dvbid/westnile/surv&control.htm
“Data are being collected on a weekly basis and are reported for the following five categories: wild birds, sentinel chicken flocks, human cases, veterinary cases, and mosquito surveillance….”
Pennsylvania’s advice on handling and reporting, as a good example:
http://www.westnile.state.pa.us/animals/deadbird.htm
Any bats? They also show up under windmills; procedures to tell what killed an animal found near a turbine are worked out pretty well. Some die not from striking the blades, but from pressure changes around high speed vanes.
The newer turbines run much slower so don’t cause nearly as much damage either from strike or from pressure change.
http://www.google.com/search?q=wind+turbine+low+speed+bird+bat
http://www.scientificamerican.com/article.cfm?id=wind-turbines-kill-bats
Mike Flynn wrote @ #450:
“So, just to ask again, when the surface radiates OLR, does its temperature drop, or not?”
RodB answered that well, I thought, and Adelady had an elegant comment, too. But let me say it another way.
“So, just to ask again, when the surface radiates OLR, does its temperature drop, or not?”
Yes. But without a “greenhouse capable” atmosphere that OLR is immediately gone for good. With one, a portion of it is received back as downwelling IR, sky radiation, call it what you will. Hence, the surface cools *less* with an atmosphere, not more.
In other words, our atmosphere doesn’t cool, as you think, but rather moderates cooling. (cf. Hank’s post re the mean surface temp of Luna.)
And building the damns necessary for backup generation through hydroelectric leads to: cutting down many trees, (deforestation) disrupting local animal and aquatic life, displacement of indigenous peoples like the Kayapo in Brazil for example, and people in India during the implementation of the Sardar Sarovar too.
Deforestation leads to erosion of the soil and less natural carbon capture.
Now on the plus side hydrolecric has a life expectancy of 100 years compared with 20 for a wind farm and 30 for a coal plant.
Three kinds of hydroelectric applications exist:
Large Hydroelectric Power (LHP) Small Hydroelectric Power (SHP) and non-renewable pumped storage plants.
SHP is the most applicable and it has a low ecological impact profile but it also is limited to geographical availability to actually implement.
LHP is more grand scale with more potential but greater risks and concerns like:
stopiing nutrient rich silt from getting to the downstream land, like in Egypt with the Aswan dam which has led to havign to use far more fertilizer in river based agriculture. Aroudn 25% of sediment flux that should be transported to coastal areas is kept behind the dams which results in more coastal erosion.
Another issue is that the viability of hydrolectric power is dependent upon the local environmental climate. Vast changes can reduce the water supply significantly.
Most locations that are acceptable for hydroelectric are in very remote areas and therefore the power needs to be transmitted over long distances.
LHP apps do impact the environment and they do this in one manner as so: the lack of sediment, rotting vegetation making the water more acidic, too much aeartion or too much C02 or CH4 in the water.
LHP’s can bring about economic growth and also death to indigenous cultures.
Oof course these conversations are important. Another issue is the damns may last far less time than the predicted 100 years or so due to silting which causes a reduction in the volume water available which can block the turbine intakes.
Further deforestation is produced by displaced people who need more grazing land.
These are not hard things to find out. Many if this is taught in elementary school.
It is amazing how the focused on the birds I can get you guys to be when it is irrelevant in your minds but you will not address the issues of hyroelectric, nuclear, wind turbines and solar:)
http://www.youtube.com/watch?v=oRxpwFfWD2c: Maybe..? And no I am not deferring to an Obama or big poli sci discussion.
Interesting:
http://www.greenoptimistic.com/2010/09/18/organic-solar-cells-greener-siliconbased-rochester-study-reveals/
These two links explain organic based solar in a more straighforward manner than my previos scholar links so if read we can be closer to on the same page.
http://www.i-sis.org.uk/OSP.php
http://www.sciencedaily.com/releases/2010/04/100409105357.htm
http://www.popularmechanics.com/science/energy/solar-wind/4306443:
\Even if the solar cell market were to grow at 56 percent a year for the next 10 years—slightly higher than the rapid growth of the past year—photovoltaics would still only account for about 2.5 percent of global electricity, LBNL researcher Cyrus Wadia says. \First Solar is great, as long as we’re talking megawatts or gigawatts,\ he says. \But as soon as they have to start rolling out terawatts, that’s where I believe they will reach some limitations.\
Even the current rate of growth won’t be easy to sustain. Despite the buck-per-watt announcement, First Solar’s share price plummeted more than 20 percent on Wednesday, thanks to warnings from CEO Mike Ahearn about the effect of the credit crisis on potential solar customers—as much as 10 to 15 percent of current orders might default. He recently told analysts in a conference call that \as good as things look for the mid-term and beyond, the short-term outlook for the solar industry in our view has never looked more difficult.\ (A transcript is available at SeekingAlpha.)\
447 (Jacob),
You keep getting lost in the details and missing the point. You seem to spend a lot of time lecturing other people on your knowledge of things, without recognizing that others may easily know as much or more than you, and you have no way of knowing whom you are talking to.
Don’t for one moment assume that you are smarter or more educated than others here.
Beyond this, the 100 or 200 years or 500 years, supplemented or not by synthetics, is not the point.
The point is that even without climate change, fossil fuels will eventually run out. If your position is that fossil fuels are necessary to civilization, since they will run out, then civilization will end in some relatively finite time frame. There’s no getting around it by spending two dozen paragraphs avoiding it with details.
That’s obfuscation.
If civilization must have fossil fuels to survive, then civilization is doomed. If fossil fuels are not necessary, then there is no reason to put off the pursuit and introduction of renewable energy sources.
This, of course, all goes back to climate change. You seem to say “we must have fossil fuels” and therefore we shouldn’t waste time with renewables and therefore climate change is an insurmountable problem. But that argument is DOA because fossil fuels must run out, in which case there’s no reason not to aggressively pursue renewables today, as a cure for the climate issues caused by fossil fuel use.
Hank you really ought to read the references you place at least on the first page:
http://www.yourpublicmedia.org/content/wnpr/protecting-birds-and-bats-wind-turbines
The wind industry loves low numbers like that, and points to estimates that turbines kill only 60,000 to 100,000 birds a year, a tiny fraction of the millions killed by other common hazards like cars and household cats.
But many projects are proposed for prime bird habitat including ridge tops and on the coast all the way from Rhode Island to Maine. And that could impact more birds.
“We know that there is potentially large mortality,” said Ken Rosenberg, Director of Conservation Science at the Cornell University Lab of Ornithology.”
People seem tp forget about ecology and biology and to think I was only half serious about the birds but thanks to Hank we see some very experienced experts are concerned. Thanks Hank for the references.
“
And yes Hank I see that they are changing the blade speed [edit]
[Response: Sorry folks. This particular discussion thread is now closed. Way OT. Readers interested in discussing the relative merits of various alternative energy sources can find plenty of other sites to discuss this at. Not here please, and certainly not in the comment thread of this article. -mike]
[Response: Looks like Mike just beat me to it by a moment but… Please stop wandering over every conceivable topic under the sun and focus on the topic of this post, only.–Jim]
No problem Mike and Jim. I made my point.
I think we need more data to support an attribution to the current volatile weather and heat waves.
[Response: whatever. -mike]
“The links I provided show why we [edit–no more on this please]
How about Wetter and Wetter instead of Warmer and Warmer? That web site:
http://www.ncdc.noaa.gov/extremes/records.php?ts=daily&elem=prcp&month=9&day=2&year=2010&sts%5B%5D=US&submitted=Get+Records#recs
shows a lot of record busting rainfalls this year. Some of the new records are almost 4 times the old record! I would have expected a new record to be a few % more than the old record. Has anybody done any statistics with rainfall records to prove GW?
Thanks for showing me that web site.
MF 450,
You are looking at longwave radiation in isolation. If that were the only process operating, the surface would steadily cool. But it isn’t. Incoming and outgoing energy balance over the long run–to be precise, you have 161 watts per square meter of sunlight and 333 of atmospheric back-radiation warming the Earth, while 397 W/m^2 of outgoing longwave and 97 W/m^2 of convection, conduction and latent heat transfer cool it.
161
333
—
494
97
—
397
JM 461: [edit-no more on this topic please]
Re# 472: Great Global Wettening.
Have you seen any comparable data for the whole globe?
It might be easier to infer along the route:
global => global
than
regional => global.
> has anybody done any statistics
Yes. Search finds plenty. Just as examples:
http://www.epa.gov/climatechange/science/recentpsc.html
http://scholar.google.com/scholar?hl=en&q=rainfall+records+climate+change&as_sdt=2000&as_ylo=2010&as_vis=0
Speaking of “warmer”, I don’t suppose RealClimate could do a post on the differences between RSS, UAH, and the Q. Fu work at U Wash regarding tropospheric temperature trends? Perhaps with some references to radiosondes, the Douglass-Santer debate, and such thrown in?
-M
@moderator(s)
I notice that my last post to “Warmer and warmer” didn’t make the grade, and was moderated out of existence as far as I know.
It was flagged as awaiting moderation, so I am assuming it got past the spam filter, which caught me in the past.
I was merely responding to people who either misunderstood what I was trying to say, or put expressed their views in a manner which I failed to understand. Possibly my observations about rapid desert cooling and ice formation when the atmosphere is above freezing on a clear night met with scepticism, or even outright rejection.
These are personal observations, and I wonder what the causes are. As you are aware, I didn’t finish secondary school, so I would appreciate physical explanations of some things that don’t appear to follow from some of the statements of posters who claim to be more knowledgable than I.
The problem is that comments such as “Crack a book”, “Puh-leaze”, “flat-out wrong”, “nonsense” and all the rest, don’t tell me how a cooler atmosphere can heat a warmer surface. Even ice at 20deg K radiates heat, as far as I know. However, it can’t add heat to ice at 250 deg K as I understand it.
If my solar hot water collector is at 70deg C, surely it would heat the atmosphere above it, not the other way round. I am going mad here. If you don’t want to help, that’s fine.
Maybe my post went astray for some other reason, in which case could you let me know.
Actually, I probably haven’t got many years left, so it is relatively unimportant. I remain curious, nevertheless.
Kind regards,
Mike Flynn.
[Response: Don’t know what happened there Mike but sometimes posts do apparently vaporize. There are a number of people here who can answer your questions. One point though wrt your question is that it’s not so much an issue of a cooler thing heating a warmer thing, but rather of the warmer thing not losing as much heat as it otherwise would if the cooler thing weren’t there.–Jim]
Mike Flynn (478) — a little twist on Jim’s comment. I think you might be confusing heating with net heating. If a body A at 20C radiates energy, cooling itself, and that energy is absorbed by body B which is at 100C, it is going to heat body B. But body B is radiating out more energy and cooling itself more than body A is heating it. So body B is cooling but not as much if body A is in the picture. The two radiation signals do not collide and net out in the path between the bodies.
For fun body A is probably absorbing body B’s strong emission and, even though cooling in its own right, is in net, with absorbing B’s radiation, heating up. Eventually, if all there is is body A and body B, and there are no other heat sources, they will get to the same temperature — though continue to exchange radiation, more or less.
Some of the escaping infrared radiation from earth will make it to the sun and heat it — just not very much (actually damn little, but some.)
Calculating the precise amount of heating/cooling is considerably more complicated than the above describes, but the idea is correct.
Mike Flynn (478)–
You should read our paper on this subject of hot-cold radiative heat flows. We provide some basic examples to show that cold objects can in fact heat warmer ones with emphasis on the sometimes cited “colder atmosphere cannot warm the warmer surface” line of reasoning in connection with the greenhouse effect.
Mike Flynn,
It would appear that you are identifying an “object” and noting correctly that it radiates blackbody or thermal radiation. However, you are neglecting that the “environment” in which the object is found also radiates. If the object radiates more energy than it receives from the environment, the object cools. If the reverse, it warms. If energy in equals energy out, we have equilibrium (e.g. constant temperature). Does this make sense?
Note that the Universe itself radiates at its characteristic temperature o 2.7 Kelvins.
Mike Flynn,
Try here:
http://bartonpaullevenson.com/JJandJ.html
Uh, oh.
http://johncarlosbaez.wordpress.com/2010/10/05/power-density/#comment-1702
@moderator
Thank you. I will repost the missing post. WRT your comment about warmer/colder, I have no problem with insulation, or “cold” bodies radiating heat. Everything above absolute zero radiates heat, as far as I know.
Many apologies in advance if I appear dense, but the surface can only warm by receiving heat (I think). I believe heat flows from hotter to cooler — not the other way round (net energy transfer — I am aware that gas molecules have different energies, and Maxwell’s demon is happily asleep.)
Correct me if I am wrong, but the surface warms due to radiation from the Sun. The general atmosphere not nearly as much (being much less dense, (even if some gases may have a higher specific heat than water, say), at least in the troposphere.
So, in general terms, excluding fluke weather conditions, the atmosphere above the surface is cooler than the radiantly heated surface. My question remains — how does any part of the cooler atmosphere warm the warmer surface?
If the CO2 in the atmosphere has a temperature of say 35 deg C (my locality daytime screen temp.), how does it reradiate heat to the bitumen road out front with a temperature of 50deg C? By the way, my solar hot water system collector gets far hotter — so the temperature differential is even greater.
@adelady 455.
Can I point out that every place on Earth receives very nearly half the available insolation during a complete orbit round the Sun, because the Earth is more or less spherical and rotates around 365 times during its elliptical (more or less) orbit round the Sun. Hence night/day, diurnal variation etc.
Can I ask you to explain in fairly simple terms how heat flows from a cooler point (the atmosphere) to the hotter surface — say my solar hot water collector (above 80deg C AFAIK)?
In relation to a cooling Earth, I believe the Earth stated life as a fiery ball. It is quite a lot cooler now — understandable, as it sits in the near absolute zero of space cooling down. The best efforts of the Sun to maintain the molten state appear to have failed.
@ Hank Roberts 456
Thanks for the link. No thought experiment needed. That’s what I thought, but sorry if I expressed myself poorly. Maximum temperature observed on Earth (Wikipedia) 57.8 deg C. Maximum possible using Moon as proxy — around 100deg C, according to your link.
Can you assist in pointing out to me how I have misunderstood the laws of thermodynamics. I thought heat flowed from warmer to colder — otherwise you could suck the considerable heat energy out of an iceberg, and use it to boil water for your cup of tea.
@457 Rod B.
Yes, I think I understand the mechanism. My question remains as to how the raised CO2 levels in the atmosphere increase temperatures at the surface. If the CO2 was warmer than the surface, then the surface would warm. But the CO2 seems to be at the same temperature as the other gases with which it is mingled — around 1 CO2 molecule to 2,000 other gas molecules (apologies if I got it wrong). So how do the CO2 molecules transfer heat from themselves to a warmer body (the surface)? I obviously don’t understand something.
Just talking about the CO2 radiation component — this is what is supposed to causing increased temperatures, above those that would otherwise be the case.
And yes, I have read Weart’s summary. You might like to point out where he refers to heat being radiated from a colder object to a warmer one (and actually raising its temperature). I seem to have missed that bit.
@464 Kevin McKinney.
Thank you for confirming my thought that when an object loses heat energy by radiation, it cools. I also agree that without anything to stop it, the OLR is gone for good (ie flowing from warm to the near absolute zero of outer space, never to be seen again). Hence, very rapid cooling in desert regions away from the sea on cloudless nights, during the appropriate season.
If the atmosphere doesn’t cool, as you tell me, then the desert surface couldn’t drop below zero at night, after reaching “bloody hot” temperatures during the day.
@All.
I see even NASA have 117% of incoming solar radiation leaving the earth. Yes I know, I’m stupid — well, I never finished high school. I just can’t see how you can radiate away 117% of the radiation you receive. I also can’t understand the “colder atmosphere” warming the “warmer surface” during the day to “create” the extra energy.
I understand the atmosphere acting like a blanket — slows both the cooling and heating of the surface, to “smooth out extremes”. But a blanket contributes precisely zero heat energy. A corpse or a block of concrete wrapped in a blanket eventually reaches thermal equilibrium with its surroundings. No different from without the blanket, given time.
I understand that GHGs can absorb IR radiation, and warm up. However, as soon as they are warmer than their surroundings, they radiate heat, until they achieve thermal equilibrium with their surroundings. Bearing in mind that the heat energy they absorbed never hit the ground, the surface did not get as hot as it would have without the atmosphere. Look at the moon.
“Aha”, you say, “but the GHGs reradiate the incoming radiation to the surface.” Yes, if you can overcome the problem of moving heat from a colder object to a warmer one, and assuming 100% efficiency, the surface would receive as much energy as it would have without the GHGs interfering.
Alas, entropy increases. Energy conversion inevitably involves inefficiency AFAIK.
So, the GHGs don’t warm the surface quite as much as if they weren’t there. The waste heat is at a lower temperature than the radiantly warmed surface, so it can’t go there. I guess it winds up eventually in outer space.
I understand the concept of radiation “windows”. But if you block any type of radiation, you are transmitting less than 100%. Retransmitting it later won’t help.
I understand the concept of insulation. Once again, it doesn’t work to provide any additional energy. And by the way, the best insulator I know of is a vacuum surrounded by shiny surfaces — Dewar or vacuum flask. Maybe there are better ones, but I don’t know about them.
I seem to have agreement that the OLR lowers the surface temperature, as it flows to a cold sink. How does the radiation from the CO2 in the atmosphere raise the temperature of something warmer than itself?
Sorry, but all my searches have proved fruitless in respect to heat moving “uphill”.
Live well and prosper.
Addendum — sorry but post apparently vanished for a while.
@479 Rod B
I agree. The warmer body does not get any warmer due to the cooler body’s heat radiation. And yes, they both reach the same temperature — at which point they will both be radiating equal amounts of heat.If your bodies have the same mass, and are enclosed by a perfect insulator, I figure that the final equilibrium temperature would be (293 + 373)/2. No energy change in total, no gain in energy (net) by the warmer body at any time. Pardon me for assuming a perfect insulator, and no losses on the energy exchange.
@480 Chris Colose.
Thank you. In your paper I saw some infinite planes, infinitesimally thick surfaces and so on. Your statement “. . . examples to show that cold objects can in fact heat warmer ones . . .” seems to relate to the fact that a body will cool slower if insulated, repeated in different guises. I agree that colder bodies will radiate heat, which will reach warmer bodies. I have yet too see an example of the temperature of the warmer body rising, as apposed to cooling more slowly. Could you please direct to the example in your paper that supports this? I seem to have missed it.
@481 Ray Ladbury.
Thank you for your post. My point precisely. So if my solar hot water collector is at 70 C +, it has received (obviously) energy from a warmer object, not a colder one. The only object that I can observe that might do this is the Sun, and lo and behold, when a cloud obscures that source, the collector surface temp starts to drop, and stops when the incoming energy equals the outgoing energy again.
When the Sun is switched off at night, the temperature starts to drop, and continues to drop (on a clear, windless night), until the Sun provides more energy, and the temp starts to rise.
And so on. What people seem to forget is that any energy absorbed by the atmosphere, is energy that didn’t reach the surface directly. This insulating effect probably explains why the maximum temperature on the earths surface is demonstrably less than that of the Moon. The reverse would apply at night, which is why the dark side of the Earth doesn’t lose its heat more quickly than it does, like the Moon.
@482 BPL
I’m sorry, but your granite blocks thought experiment seems a bit odd. You have a six sided cube emitting 221.5 W per side (m2).
That would appear to be radiating 6×221.5 W or 1329 W for an internal input of 221.5 W. Maybe I missed something. In any case, if you heat up two objects by supplying energy (say internal heating elements) it is likely that both will increase their temperature, given enough heat. If you turn them both off, they will both cool. My guess is that it will take a bit longer than a single block, because they are radiating at each other that radiation that hasn’t escaped to another energy sink (insulation, outer space whatever). However, I note that your granite blocks also may have been transmuted into infinitely thin 1 m2 surfaces, which would explain the heat discrepancy. In this case, however, it is probably better to have a perfect mirror on the ‘back” side.
Now you might care to examine what will happen if you turn the “internal” electrically powered heat source of A, B, or both off. Infinitely thin means infinitely small thermal mass. Turning both heat sources off will result in absolute zero temp for both plates in an very short length of time. Turning one or the other off will reduce the temperature of the one without internal heat to that resulting from the radiation of the heated plate. In no circumstances can the colder “heat” the warmer (that is increase its store of internal heat energy).
If you have two objects being heated internally, the temperature of each will rise until the net radiation of each equals the net energy input of each. The fact that both of your objects increased their temperature, and thereby the total heat energy, would indicate that external energy was supplied — and indeed that seems to be the case. So, sorry Barton, but it seems that you have increased the energy in both blocks by some method that gets around the concept that energy can neither be created or destroyed.
@all.
Many apologies. However, all posters who responded seemed to be saying different things. I was just trying to make the point that heat radiation seems to reach the surface and heat it without more than about 30% being “delayed in transit”. The same for outgoing radiation from the surface on a clear night. Such “net” radiation surface vs atmosphere etc., seems to explain simply why things (the Earth, the Moon) warm during the day, and cool at night.
One last observation. The sky is blue, and clouds are white, and if I move into the shade I cool down somewhat, if the Sun is too bright. These three facts should indicate whether cloud emit or reflect IR, what wavelengths are absorbed by the atmosphere, and what wavelengths allowed through, and whether IR radiation from the Sun reaches the ground without the assistance of the atmosphere.
Obviously, the Earths surface will warm after glaciation. If another glacial period comes, the Earth will have cooled. In the meantime, the Earth continues to cool overall. No longer molten. So I can accept simultaneous cooling and warming — it’s pretty obvious.
Just curious about heat.
Live well and prosper.
Re 484:
Mike,
I’ll take a stab at answering your concerns. But for full disclosure, I am an engineer, not a physicist or climate scientist, so I hope you and the excellent cadre of regular commenters will be gentle if I write something really boneheaded.
In understanding the physical processes of climate it is important to keep in mind the distinction between energy and heat. The terms are not synonymous. An object can absorb radiant energy and heat up, or radiate energy and cool. Radiative heating and cooling is a different process than heating or cooling through conduction or convection.
A simple example that probably everyone reading this is familiar with is a microwave oven. Ask yourself – how can this room temperature appliance heat food without heating up itself? And without violating the 2nd Law of Thermodynamics. Even a small microwave oven can heat things to the point of combustion (sadly, I speak from experience). Unlike a conventional oven which heats food through conduction and convection, a microwave oven generates radiation at a frequency strongly absorbed by water and organic molecules. That energy doesn’t become heat until it’s absorbed by what’s being cooked. But put an empty coffee mug or glass into a microwave and they won’t heat up – because they don’t absorb the energy being radiated by the magnetron in the oven.
I believe that we are all on the same page that ALL objects radiate energy at their blackbody value. The earth radiates LWIR so let’s follow a photon after it is radiated by the ground. I believe that we also all understand that if there were no GHGs or water vapor in the atmosphere that photon would keep going until it disappeared into space because non-GHG components of the atmosphere are transparent to LWIR.
But in the real world that photon would travel some distance and then be absorbed by a GHG molecule of, say, CO2 thus raising its energy level, i.e. its temperature. That molecule can do one of two things, it can re-radiate that energy as another photon, or it transfer that energy to other molecules through collisions, thus raising the aggregate gas temperature. If that re-radiated photon reaches the earth it is absorbed and the process starts over with no net cooling of the earth.
Please note that the GHG molecule doesn’t know or care what the aggregate temperature of the surrounding gas is. If it absorbs a LWIR photon it is likely to re-radiate a LWIR photon. So when the earth radiates LWIR energy into a cold sky containing GHGs, a percentage of that energy is going to return as re-radiated LWIR, warming the earth as it is absorbed. There is no violation of the 2nd Law in this process.
Does this answer your concerns at all?
Mike Flynn seems (TLDR) to be conflating energy and causality.
When I cook, one might say I’m causing the temperature of my natgas oven to increase even though I am colder than the oven. And that’s not simply because I’m dissipating electrical energy as in Philip Shaw’s example (cheater!). Energy flows from the burning gas to the oven to me. Yet, if I wasn’t there, the oven would be colder because it’s in my nature to cook and it’s not in the nature of the oven to turn itself on.
Energy flows from the Sun to the surface to the CO2 (on average). Yet the CO2 can be said to be causing the surface to warm because it would be cooler if the CO2 wasn’t there. The reason is that it’s in the nature of the CO2 to radiate IR in all directions while it’s not in the nature of the surface to emit at wavelengths as short as the Sun.
Phenomenons can have any number of causes without contradiction. That’s because causality is derived from counterfactuals. The Sun is warming the earth and so is atmospheric CO2. But the net flow of energy only goes one way because it’s derived from a comparison: only one of two flows can be larger than the other.
So, while you can infer the direction of a causality from the net flow of energy (the surface warms the CO2 because it’s hotter and hotter objects radiate net energy to colder ones), you can not infer that no causalities are flowing in the opposite direction.
> GHGs can absorb IR radiation
That’s right
> Bearing in mind that the heat energy they absorbed never hit the ground,
That’s one place you went wrong
Most sunlight is in the visible range.
Sunlight hits surfaces (water or earth) and warms them.
The energy of all those photons of visible light becomes heat.
The surface radiates photons at that temperature; those are infrared.
So — incoming visible; outgoing infrared.
The visible coming in goes through the atmosphere.
The infrared from the surface gets absorbed, warming the atmosphere.
quam boderve
er, those last 2 words were meant for ReCaptcha, sorry.
@486 Anonymous Coward.
Not at all. You may not have understood me when I said that all objects above absolute zero radiate energy as heat.
If they didn’t they would be at absolute zero — that may even be the definition of absolute zero (haven’t looked it up).
My response to some later posts has definitely been caught by the spam filter, so I will reiterate for you.
Net heat energy exchange proceeds from the warmer object to the less warm (or if you like from the less cold to the more cold) – until thermal equilibrium obtains. I agree that having an atmosphere (whether it contains CO2 or not) causes the surface to warm up more slowly and cool more slowly than would otherwise be the case.
Your statement that surface would be cooler if the CO2 was not there, doesn’t seem to agree with the observation that the surface of the moon, without the assitance of CO2, gets hotter than that of the Earth.
I suppose you are going to tell me that my solar hot water collector temperature of 70C plus, would be cooler if there was less CO2 in the atmosphere.
Now maybe you can deconflate energy and causality for me, and provide me with the counterfactuals from which I can infer the causality that removing heat energy from the Earth to warm the CO2 leave the energy content of the Earth unchanged. My understanding is that if you remove energy, the body will cool.
If, as you state, a phenomenon such as the Earth cooling over the last 4.5 billion years or so, can have any number of causes without contradiction, I suppose I will have to accept an assertion that the Earth has been bathed in negative heat energy for that time. If the energy changes to positive, and you can’t contradict this, according to you (as it is a cause), then you have global warming.
I certainly haven’t said that a cooler body is not radiating heat — I’m just saying that the cooler body cannot increase the net energy of the warmer. No how, no way, not ever.
A perfect insulator would radiate back to the emitter 100% of the emitted radiation of all wavelengths. And the emitter’s temperature will rise? However, I will not contradict you if you prove with a computer model (or by any other means) that a perfect insulator can radiate more than 100% of the radiation it receives.
Good luck with your cooking. I am glad you have realised that it is not in the nature of ovens to get any warmer than the surrounding environment by themselves.
Live well and prosper!
@487 Hank Roberts.
I wonder if you can quote a source for the percentage of energy for the various wavelengths emitted by the Sun?
My information is that less than 50% is emitted as visible light.
If you follow what I have been trying to point out, it doesn’t matter what the relative energies or wavelengths of the insolation are, as long as we both accept that a body emitting energy has its temperature lowered.
Go and stand in bright sunlight, and you will notice that quite a lot of IR seems to travel in a straight line from the Sun. Stand in the shadow of the top of a tall flagpole (or similar) on a cloudless day, and you will notice that the incoming IR reduces, regardless of the fact that you are still being bathed in radiation from the blue sky.
I hear what you say. I am not sure how the atmosphere blocks outgoing IR during the day, but not at night.
Or does all the CO2 in the atmosphere only absorb LWIR in the presence of sunlight?
You might also like to give a reference that shows that IR radiation leaving a body doesn’t lower its temperature.
Live well and prosper!
Mike, the elementary answers are in the FAQ and Weart’s book.
Set aside what you think you know.
You’re making assumptions that don’t help with your questions.
Sunlight “emitted” differs from sunlight at the surface.
http://www.google.com/search?q=spectrum+sunlight+ground+level
Sunlight at the surface heats the surface.
The surface radiates warmth — infrared.
This happens day and night. Why do you imagine it doesn’t?
You can get an infrared thermometer and look for yourself.
http://mynasadata.larc.nasa.gov/P18.html
Read the weather balloon/altitude part to understand what it measures.
Yes, you can feel infrared, and see visible light. How do you know how much? You can find this kind of information for yourself.
What the sun produces:
http://www.chemistryland.com/CHM130W/Final/VisibleLightSpectrumPlus.jpg
But look at what the solar spectrum is at ground level — different, eh?
You know how to find this stuff.
You have a reference librarian near you, or a school librarian — that’s a good place to start asking how to find answers to these kind of questions.
Extremely elementary explanation and animation:
http://earthguide.ucsd.edu/earthguide/diagrams/greenhouse/
Mike Flynn, this may explain where some of what confused you originated:
http://scienceofdoom.com/2010/04/05/on-the-miseducation-of-the-uninformed-by-gerlich-and-scheuschner-2009/
Oh, I should’ve checked. Mike Flynn has also been having this conversation at
http://scienceofdoom.com/2010/10/02/absorption-of-radiation-from-different-temperature-sources/
Mike Flynn@489
You say “…I certainly haven’t said that a cooler body is not radiating heat — I’m just saying that the cooler body cannot increase the net energy of the warmer. No how, no way, not ever…”
I won’t even try to pretend I’m not over my head, BUT, I used to believe:
A radiated photon has no memory of its radiator’s temperature. A photon radiated from an atmospheric molecule can go in any direction, including down (back to earth). If a photon is absorbed wherever, it will increase the internal energy of the absorber – until the absorber gets rid of (some of) its energy by radiation of photons and/or transfer of mechanical internal energy by conduction. Heat is not energy, it is the transfer of energy.
Given all my listed beliefs, I can not understand your “no way” statement.
I expect my biggest problem is that (to me) heat is not energy, it is the transfer of energy.
You might want to reword your “no way” to distinguish between say “heat” and internal (e.g.mechanical) “net energy”. In my world, confused though I may be, there are many internal forms of mechanical energy. I know of no particular restrictions (laws) constraining internal mechanical energy.
If this is any help, you are welcome. If it is not helpful, I apologize for any waste of your time.
John
Mike Flynn@489
Schwaum’s Outine – Thermo for Engineers suggests trying to explpain how a microwave oven, (not a gas or electric oven) works.
john
@491 Hank Roberts
I am sorry to don’t want to provide me with a reference for your statements. Where did you get your percentages — Weart or the FAQ’s? Where?
1. I am aware the surface radiates heat day and night. My point exactly.
2. You tell me to find out the information about the percentages of different wavelengths of light myself. “How do you know how much? You can find this kind of information for yourself.” I know about school librarians, reference librarians — I asked you. If you don’t want to tell me, fine. I understand.
3. @492, 493,494
I don’t believe I’m confused about the laws of thermodynamics to the point where a cooler body can heat a warmer body (in any meaningful sense above the quantum level).
And yes, I have read some of the “references” that you have tossed at me. You might have the courtesy to establish what I have read, and what I have not in future.
Just to have a tiny nit pick at Forrest Mims, I suggest you conduct the following experiment. Obscure all the glass on your motor vehicle with aluminium foil. Park car in blazing sun. Ensure all windows up, doors closed. Should be totally dark inside. Take temperature readings until the rising temperature forces you out. Repeat experiment without the foil.
Now tell again why I should believe the statements on the web site to which you referred me are believable.
But thanks for your efforts.
@495John Peter
I cut and pasted this from Wikipedia
“One study showed that several popular textbooks used language which implied several meanings of the term, that heat is the process of transferring energy, that it is the transferred energy, i.e. as if it were a substance, and that is an entity contained within a system, among other similar descriptions. The study determined it was not uncommon for a combination of these representations to appear within the same text.[8] They found the predominant use among physicists to be that if it were a substance.”
Obviously, we differ in our usage of the term “heat”. I must admit I assumed that others would adopt what appears to be “ . . .the predominant use among physicists.”
Given your definition, I will restate, slightly differently, my statement.
A body at a given temperature cannot transfer energy to a body at a higher temperature in such a fashion as to raise the temperature of the warmer body in any meaningful way.
Does this suit you in your endeavour to transfer your beliefs to me?
I am aware there are many forms of energy. Even matter is merely concentrated energy — hence e=mc2 and nuclear power. I am not sure what you mean about no particular restrictions (laws) constraining internal mechanical energy.
I consider that the “Laws of thermodynamics” apply to all forms of energy.
Please excuse me if I wasn’t clearer originally. I am talking about “real” objects, no “Maxwell’s Demon” and so on. Obviously, it is possible for all the high kinetic energy molecules in a container of gas to simultaneously rush to one end, and all the low energy molecules to rush to the other.
Now we have a local decrease in entropy – and the “hot end” could transfer heat to the “cold end” of another such container. However, I repeat “No way.”
Heat travels from hot to cold. Or so I believe, until you can demonstrate otherwise.
I await a convincing demonstration (real rather than hypothetical).
Not terribly helpful, but thanks anyway.
Live well and prosper!
John Peter,
Mike Flynn’s error is more simple than that. In order to understand it, you must take your attention away from what the illusionist is pointing to. Look at the implied counterfactual, not at the thermodynamics involved.
A body has no “net energy” of course. It has energy. Net energy refers to the difference between two (or more) flows. But let’s not be pedantic.
What’s important to understand is that any insulation, even 1% “efficient”, causes what it insulates to warm after application. While net energy flows from the object to its insulation, some energy flows from the insulation to the insulated object, causing it to warm.
Mike is correct that, on average, the energy flows from the surface to atmospheric CO2. At night, in the absence of solar radiation, the surface cools. But, if you take away the CO2, the surface would cool even more.
Mike’s implied counterfactual is that the surface would not radiate the energy it transfers to the CO2 if the gas wasn’t there. This is of course unphysical. The surface would radiate energy towards space anyway. The CO2 only beams some of it back towards the surface.
The surface of our moon is on average colder than the surface of the our planet. Some of the difference is caused by non-radiative processes (a transparent atmosphere would cause the surface to be somewhat hotter than in the no-atmosphere counterfactual) and some of it is due to the so-called greenhouse effect.
Mike Flynn,
You need to look at this in terms of the physics of what is actually occurring. Yes, a warmer body will radiate more energy than a cooler one. However, in the climate system, how much heat a particular component radiates is not the relevant quantity. The relevant quantity is how much of the radiated energy escapes to the inky blackness of space. If the radiation is absorbed by the atmosphere, it stays in the climate. Moreover, because the atmosphere is relatively cooler than the surface, more of the energy absorbed by CO2 gets transferred to nitrogen molecules when the CO2 collides with the nitrogen. What matters is net energy flows. Keep your eye on that. Diagram it out for yourself. Don’t let yourself be fooled by an overly simple model that is after all a product of your own thinking, not a reflection of reality.
Jeez. Mike FLynn: “I don’t believe I’m confused about the laws of thermodynamics to the point where a cooler body can heat a warmer body”
You are confused if you think that has anything to do with why CO2 is warming the planet. What does your knowledge of thermodynamics tell you will happen if you’re lying in bed shivering. You have a blanket in your closet. The blanket is initially cold since it is in equilibrium with the air temperature in your room. You put it on top of all the other blankets on your bed and climb back in. Will you be warmer or colder after the new steady state is reached? Or does your knowledge of thermodynamics tell you not to put on extra blankets when you’re cold?
> aluminum foil
Right experiment. Wrong conclusion.
Compare the result if you use a “cool roof” white paint instead.
The difference is “emissivity” of the material — how effectively it radiates away absorbed heat as infrared, compared to transferring it into the surface.
http://www.google.com/search?q=emissivity+polished+aluminum+sunlight