Recently, I received multiple requests to discuss a paper, due to appear in Journal of Geophysical Research (JGR-atmosphere), that has been presented in the media just before the Bali conference and the Nobel Peace prize ceremony here in Oslo, Norway. The paper concludes that the warming measured over land is most likely exaggerated due to non-climatic effects, and it presents a regression analysis suggesting that the real (climatic) global mean temperature trend should be ~50% lower over land.
So, are the surface temperature trends inflated? This new paper by McKitrick & Michaels (henceforth ‘M&M2007‘) is a followup of an earlier paper they wrote in 2004 in Climate Research (MM2004a), which I discussed in my first RC post (Are Temperature Trends Affected by Economic Activity?) and in a commentary in Climate Research (Benestad, 2004).
So what’s new? Let’s backtrack a little and recount some of the previous arguments.
One of my main concerns then was that their analysis had not taken into consideration the dependency between the data points, as the temperature exhibits non-negligible spatial correlations. Furthermore, data from the same country were compared with the same national value in terms of economic indices. It was a bit like doing a poll by asking 10 people the same question 100 times and then claiming that it’s a survey with a sample size of 1000.
In 2004, M&M2004b said they were unaware of any paper in the refereed applied climatology literature that had performed a test where half the data was excluded when doing the model calibration and the rest was left for model validation. I guess that they were not really up-to-date then, because that has been a standard approach for a long time.
But this time they have split the data sample and used a part for validation, which I suggested in my comment in Climate Research. But they have not done it properly this time, and they still do not eliminate the effect of dependency. They split the data by randomly picking points which were either used for training the data or validating the model, thus data from adjacent sites which are related will end up in the different batches for training or validation.
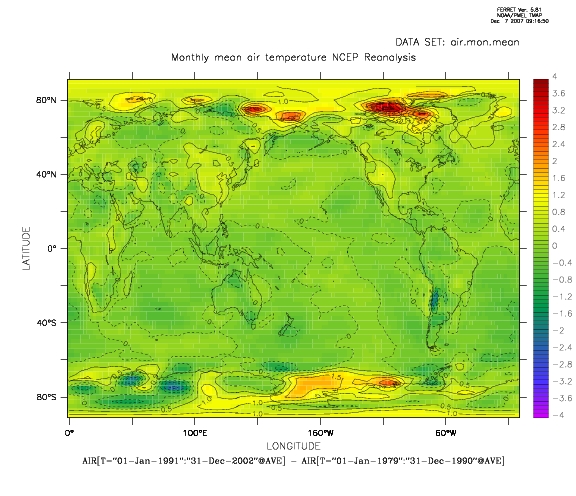
The map above shows a simple estimate of the temperature change over the 1979-2002 period (here taken as the differences in the mean over two sub-periods and the National Centers for Environmental Prediction (NCEP) re-analysis have been used instead of the CRU data), and it’s easy to see that the warming varies smoothly from location to location. In other words, the trend estimates have significant spatial correlation.
The fact that they used sea-level pressure (SLP) data from (1974) because they could not find more recent data, suggest that they still are not up-to-date. Updated data, such as the National Center for Environmental Prediction SLP, have long been available from NOAA Climate Diagnostics Center. Furthermore, a wealth of up-to-date climate data are available from the KNMI (Dutch Meteorological Institute) ClimateExplorer.
Their regression analysis appears to suffer from over-fitting, since they have thrown in a lot of variables (both ‘meteorological’ and ‘economical’) for various vague reasons.
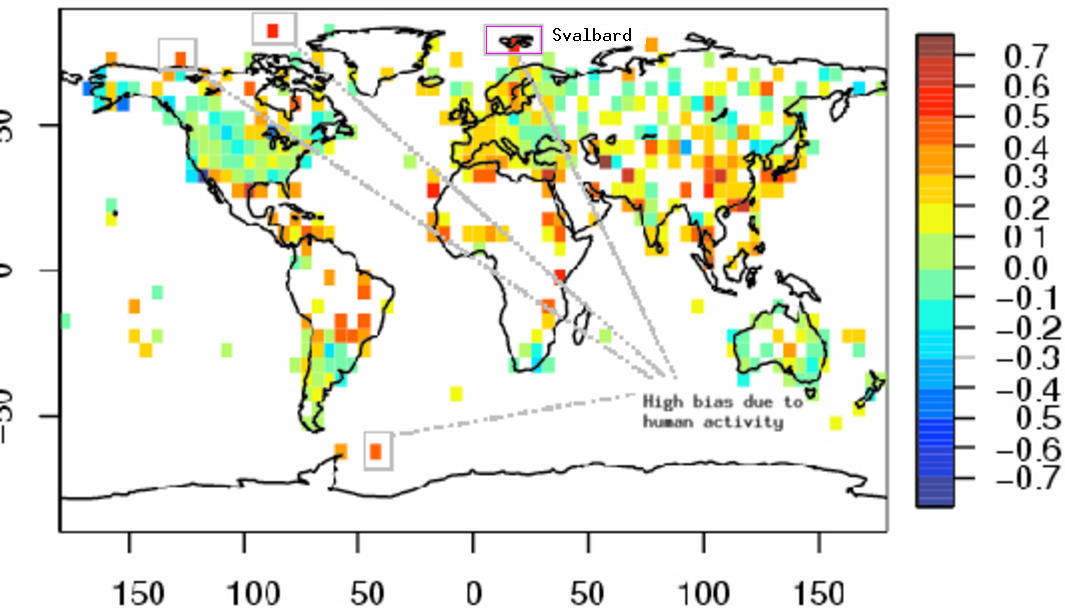
Not surprisingly, their analysis produces some strange results as a result of this shortcoming. They find that the greatest differences between measured and adjusted trends at Svalbard and other places in the Arctic and Antarctic (See marked sites in Figure below). This is not convincing. Thus, the results themselves provide examples of spurious values obtained by their analysis. Even if they were identified as ‘outliers’ (Svalbard was apparently not one), the fact that their analysis produced highest corrections for economic activity at these places suggest that their analysis is not very reliable.
The graphic below shows a Google Earth image of Svalbard, which is one of the sites marked in the map above with a large trend correction due to economic activities.
I have not examined the economic data, but it appears that M&M2007 maybe cannot win – either (i) the spatial distribution of the economic indices are equally smooth and M&M2007’s attempt to account for dependencies within each country fails to resolve the problem of dependency between the countries, or (ii) the economic indices vary abruptly from country to country and thus have very different spatial scales and structures to those seen in the warming trends. Either option suggest that their analysis may lead to spurious results, over-fit, or suffer from inter-dependencies.
I also think that M&M2007 is biased and gives an incorrect picture, as they do not discuss the fact that also the world oceans are warming up, and whether any economic activity can take the blame for that. I think it is difficult to argue that factors such as the urban heat island effect plays an important role here.
They do not mention my criticisms raised in Benestad (2004) either, which discussed a number serious concerns about their previous study; They merely state, as if it were a matter of fact, that urbanisation and economic activity has been shown to affect local and regional temperature measurements – citing their old criticised paper.
Their analysis relies on University of Alabama-Huntsville (UAH) satellite data (Microwave Sounding Unit, MSU) with a weaker global trend than others, and neglect to examine or even mention other products such as the Remote Sensing System (RSS) data. The difference between these data sets are discussed in previous RC posts (here). They reckoned that any of their results would not be contingent on the choice of MSU product, but did not test this hypothesis.
It should also be kept in mind that their analysis involved too short time series (24 years) for a proper local trend estimation, as local circulation variations (e.g. the North Atlantic Oscillation), the annual cycle, and inter-annual variations, most likely will make the analysis more difficult. Climatic time series from single locations tend to be very noisy, but a clear signal emerges when taking the global mean (by taking the mean, random noise tends to cancel to some degree).
I find it a bit ironic when people use satellite data measurements to argue that GHG is unimportant. They rely on the fact that these measurements are derived using the very same type of physical laws as those predicting an enhanced greenhouse effect due to increased GHG levels (neglecting feedback processes).
I think it’s good that M&M2007 put a focus on the problem with data paucity and quality. There may very well be some non-climatic effects contaminating the measurements, but I am not convinced by their analysis.
So in summary, I think the results of M&M2007 analysis and conclusions are invalid because
– They do not properly account for dependencies.
– They over-fit the regression.
– Their results look unreasonable.
– They “cherry pick” the MSU data that gives the lowest trend

Re #148, Ray Ladbury
Mr Ladbury, thank you for your very prompt response to my #147.
I have no problem with the natural GHG effect, but as I said, I am presently a sceptic about the Enhanced GHG effect. However, I am certainly willing to be convinced about it by sufficiently powerful reasoning. Ice cores, tree rings and the hockey stick, although interesting topics in themselves, cannot convince me. The effect of adding extra CO2 to the atmosphere is purely a problem of Physics, and so only a properly reasoned Physics approach can solve it. Whenever possible, I believe the best Physics approach must include a mathematical treatment. This is what I have tried to do, albeit very simply, in my #147 offering above.
“You are neglecting the surface of the planet, where most of the IR photons originate. Since the temperature is warmer than the atmosphere, it will emit more IR. Moreover, as a solid, the radiation spectrum will look more like a blackbody to begin with.”
I agree I did not mention the Earth’s surface, but this was for two reasons.
(a) Real Climate’s Saturated Gassy Argument
https://www.realclimate.org/index.php/archives/2007/06/a-saturated-gassy-argument/#more-455
and
https://www.realclimate.org/index.php/archives/2007/06/a-saturated-gassy-argument-part-ii/#comment-37716
accepts that all of the photons radiated from the surface, apart from those in the “window”, are 100% absorbed, either by CO2 and/or water vapour, and so no increase in CO2 can increase the surface temperature. This effect occurs at relatively low altitudes, and most of the absorbed energy is re-radiated back to Earth. This is, of course, the Natural Greenhouse Gas Effect.
(b) This last point is illustrated by the work of Kiehl and Trenberth
http://www.cgd.ucar.edu/cas/abstracts/files/kevin1997_1.html
I based my piece on the idea in this reference, showing that a power of 165 Wm^-2 is radiated out to space by the atmosphere.
In line with the SGA, I believe that this energy leaves from high altitudes in the atmosphere. I suggest that most of the photons are created in the atmosphere at fairly high altitudes by intermolecular collisions with CO2 molecules. Some of these photons will escape directly to space, but some will be absorbed by CO2 molecules.
So far, I think I’m in agreement with the SGA, and with some of your comments. However, you stated “To begin with, photon number is not conserved”. But, the number of photons escaping to space must be conserved if energy balance is to be maintained.
At high altitudes, some CO2 molecules can absorb photons of the correct wavelength, photons which derived their energy from the kinetic energy of the atmosphere. OK, so far.
From here on, my ideas differ from the SGA.
The excited molecules can then decay again to a lower energy level, either spontaneously or by collision, so emitting more photons. Some of these can go upwards and escape, but the remainder will return to the atmosphere, where they will transfer their internal energy to kinetic energy of the other gases. However, at high altitudes, more will escape than will return because of the infinite sink for photons, SPACE. This must be so, in order to maintain energy balance.
If more CO2 is now added, there will be a tendency for extra photons to be absorbed/ emitted as above, so allowing even more photons to escape. This would tend to make too many photons escape, and this would lower the temperature at high altitude. The numbers leaving would then be reduced and so bring energy balance again. Alternatively, one can take the idea from SGA that more CO2 would raise the altitude to even higher, colder levels for photons eventually to escape. This is probably the more likely.
Therefore, what we’re left with is the required number of photons escaping to space to maintain energy balance, and this occurs at still higher altitudes, presumably governed by the lapse rate.
The temperature of the atmosphere remains unchanged.
I suggest that what is happening is that only a small proportion of the total number of CO2 molecules in the atmosphere is involved in the high altitude processes, simply enough to provide energy balance. If more CO2 is added, then the proportion taking part is correspondingly reduced, so that the actual number taking part is kept constant.
This can be seen mathematically in my post #147.
This means that extra CO2 has no effect on the temperature of the atmosphere or the Earth’s surface. That is no Enhanced GHG effect.
Re #149, Arch Stanton
Thank you for your post.
“You are also right that GHGs also act to cool the atmosphere, however you are forgetting that that the atmosphere, besides being warmed by GHG absorbed photons is also warmed by convection from the surface and (likely even more important) latent heat of vaporization of water carried aloft. Heat that is recaptured by the upper troposphere when clouds condense. These 2 factors are constantly warming much of the troposphere.”
No, I’m not forgetting about convection and latent heat. These factors are clearly shown in Kiehl and Trengerth’s piece
http://www.cgd.ucar.edu/cas/abstracts/files/kevin1997_1.html
I agree that energy is certainly carried up into the atmosphere by these means, indeed this MUST be so because it is necessary for the atmosphere to be able to supply 165 Wm^-2 to space.
Re #150 Hank Roberts
A major reference.
But which part of my # 147 or # 151 do you not agree with?
AEB
> which part
“… rather a history of justifications than a history of discoveries”
AEBanner, OK, let’s think about the radiation of the planet in the absence of greenhouse gasses. It will be a blackbody curve consistent with the temperature of the surface, correct? And in equilibrium, energy in (from insolation) equals energy out (via outgoing thermal IR). Now add greenhouse gasses and what happens? IR photons in the absorption band of the ghg get absorbed. Some of the molecules so excited than radiate another photon in the same band. However, because vibrational states tend to have a long half-life, many more excited molecules relax collisionally, transfering their energy (it is partly kinetic/partly potential after all). This heats the troposphere, but less energy reaches the stratosphere now, so it cools. Moreover, some excited ghg molecules still radiate, and some of the ghg molecules will be collisionally excited and radiate. Some of that radiation will be earthbound and some outbound. The outbound LWIR is still likely to encounter another ghg molecule and be absorbed. However, as we go higher in the atmosphere, it cools, the numbers of photons radiated away is lower, and finally when we’re high enough, the ghg molecules can radiate away the energy of their excited state–but at a much lower blackbody temperature. So when we look at the spectrum radiating from Earth, we expect to see a blackbody spectrum appropriate for Earth’s surface temperature–except in the absorption band of the ghgs, where we have a big hole. This is in fact what we do see.
http://www.globalwarmingart.com/wiki/Image:Atmospheric_Transmission_png
It is the energy that’s missing from the blackbody curve that is in fact responsible for warming by the greenhouse molecules. And why should that magically stop at 280 ppm for CO2. Add more CO2 and you get more CO2 that can absorb energy in the wings of the bands. You also get more CO2 at higher altitudes (it’s well mixed even into the stratosphere), so that pushes the altitude at which LWIR in the CO2 band can escape even higher (and colder).
And if that doesn’t make sense to you, ask yourself: How likely is it that you would find a mistake when literally 10s of thousands of PhDs have looked this over and agree with the standard model for anthropogenic causation? I find when I disagree with all the smart people in the room that it is usually advisable for me to go back and check my thinking again. Happy New Year.
Re #147 where AEBanner says
“Therefore, the change in the number of photons returning to the atmosphere = 0
This means that there is no change in the temperature of the atmosphere due to increasing the amount of CO2 present.”
In general, your arguments are correct, but the conclusion is false because carbon dioxide does not warm the atmosphere in the way that you (following Ray Pierrehumbert and Spencer Weart) describe. Putting it simply, radiative balance to space is not achieved by the alteration the amount of outgoing long wave radiation. It is achieved by changes in the planetary albedo ie regulation of the incoming solar radiation. This was pointed out by G.C. Simpson 1n 1928.
Moreover, the current understanding of the greenhouse gas mechanism is wrong. In the IPCC AR4 they say that it is analogous to the effect of glass in a greenhouse, but this was an error by Arrhenius. Wood showed that the glass in a greenhouse prevents convective cooling. It does not produce radiative warming.
The warming of the air in a greenhouse is produced by the blackbody radiation from the ground being absorbed by trace gases in the air by a quantum mechanical effect, the details of which Tyndall, Arrhenius, and Wood had no knowledge.
In a greenhouse and on Earth, the air close to the surface is warmed which prevents the surface losing heat by convection. The surface then warms and radiates more intently, which heats the adjacent air producing a positive feedback. Over oceans and vegetation the addional heat is lost by evaporation, but in deserts the thin laminar boundary layer heats up to such an extent mirages are formed.
However, it is not in deserts but on ice sheets where CO2 concentrations affect climate. The concentration of CO2 sets the maximum temperature the surface of the ice can atain, since there is little water vapour at those temperatures. Thus CO2 concentration is a factor determining whether a surface remains ice covered in summer, and so whether the planet is in or out of an ice age.
So, although changing the concentration of CO2 does not affect the longwave radiation to space or the backradiation to the surface, it does affect the albeodo of the planet which is a main climate determining factor.
It is because this feedback, greenhouse gas on conduction, is not included in the models that they have underestimated the speed at which the Arctic sea ice and glaciers world wide have been retreating. It also explains why Spencer et al. have not found the warming in tropical troposphere predicted by the models. As you point out, the upper atmosphere will not warm with greater concentrations of CO2.
Re #156 Abbe\’ Mac
Thank you for your comments. There is a lot there to think about.
AEB
AEBanner, Now that I have a bit more time, I can point out some of the faults in your model.
As I pointed out above, the assumption that the number of photons is constant is completely false. Photons are Bosons–they blip in and out of existence all the time.
It is also false that fewer CO2 molecules will be excited. First, by thermodynamic arguments alone, the same proportion of molecules will be excited at the same temperature (e.g. collisionally). LWIR radiation will excite still more.
You are also incorrect that all the excited molecules will emit photons. The vibrationally excited state of CO2 is quite longlived and undergoes many collisions prior to relaxing via photon emission. This means that at least near the surface (where densities are high), the main relaxation mode is collisional, imparting energy to all the gas molecules.
Finally, your assumption of thermal equilibrium is incorrect. As long as the greenhouse gasses prevent the energy in their absorption bands from escaping, that energy goes into heating the system, and equilibrium is restored at a higher temperature.
Abbe’ Mac, Where on Earth did you read this? Whoever wrote it was either joking or seriously deluded.
Reply to #155 Ray Ladbury
Firstly, I should like to refer you to Spencer Weart’s piece entitled “A Saturated Gassy Argument”.
https://www.realclimate.org/index.php/archives/2007/06/a-saturated-gassy-argument/#more-455
This work implicitly seems to accept that carbon dioxide and water vapour can absorb 100% of the infrared radiation from the surface of the Earth. Most of this energy is radiated back to the surface. See Kiehl and Trenberth
http://www.cgd.ucar.edu/cas/abstracts/files/kevin1997_1.html
This is the natural GHG effect, and occurs at relatively low altitudes. For the purpose of easy discussion, let us consider the atmosphere to consist of three regions, lower and upper troposphere, and high altitude as in SGA.
The infrared which is not radiated back to the surface heats the upper troposhere by increasing the kinetic energy of the molecules by inter-molecular collisions.
Heat energy also leaves the surface as sensible heat, convection and latent heat, which rises into the upper troposphere where it too provides warming. Kiehl and Trenberth show that sufficient energy is provided to this region by these means for the necessary 165 Wm^-2 to escape to space in order to enable energy balance for the Earth’s system. ( Together with 30 Wm^-2 from the clouds and 40 Wm^-2 through the “window”. ) I suggest that the available energy is converted into the necessary photon form by inter-molecular collisions in the upper troposphere and at high altitudes.
Some of the photons about to escape into space at high altitudes will be absorbed by CO2 molecules even at this height, as stated in the SGA, but it is at this point that my ideas diverge from the SGA.
Quote from your #155
“IR photons in the absorption band of the ghg get absorbed. Some of the molecules so excited then radiate another photon in the same band. However, because vibrational states tend to have a long half-life, many more excited molecules relax collisionally, transfering their energy (it is partly kinetic/partly potential after all). This heats the troposphere,…..”
Unquote
You are saying, are you not, that most of those excited CO2 molecules which relax by collision are giving up their vib/rotational energy for it to become increased kinetic energy of the troposphere (upper), and so to warm the troposphere. Right? But, I maintain that the troposphere is where the energy came from in the first place when kinetic energy became (collisionally) absorbed by the CO2 molecules!!
So I’m afraid that that is a circular process and its argument gets you nowhere.
The excited CO2 molecules will decay again to a lower rotational level, either spontaneously or by collision, so emitting photons. Some of these emitted photons will escape to space as required, and some will return to the atmosphere (as above), but the temperature will be adjusted by the overall feedback system so that energy balance will be maintained.
It is important to understand that only a small proportion of the total atmospheric CO2 is involved in this process. The relevant actions are occurring at high altitudes “where the air is very thin”, after all, not throughout the whole atmosphere. This proportion is dependant on the high altitude temperature. The number of CO2 molecules involved will be set at this temperature to provide the required number of outgoing photons to get energy balance.
If now, more CO2 is added, more photons will join in the process and escape to space, so tending to upset the balance. Correction is provided by the emitting region moving to still higher altitudes, ( in line with the SGA ), where the temperature is lower, and so the emission rate is reduced, as required.
Note that the number of CO2 molecules involved must be constant, so that when more CO2 is added, the proportion involved is reduced accordingly. This is shown mathematically by the equation kb = b1 in my #147.
You have also referred to the black body spectrum of the Earth, and the expected gap in the CO2 absorption band region. The ideas I have suggested have in no way affected the natural GHG process occurring relatively close to the surface, so the spectrum should not be significantly changed.
Please refer again to my #147, and let me know which part you do not accept, and I shall do my best to explain.
Quote from your #158
“As I pointed out above, the assumption that the number of photons is constant is completely false. Photons are Bosons–they blip in and out of existence all the time.”
Unquote
I thought it could be taken for granted that I did not mean the self-same photons. I did not want to be pedantic. No, I simply meant that the energy equivalent had to be constant for energy balance. That is, the power radiated outwards, the OGLW energy, must be kept equal to the net incoming solar energy if equilibrium is to be maintained.
Quote from your #158
“First, by thermodynamic arguments alone, the same proportion of molecules will be excited at the same temperature (e.g. collisionally).”
Unquote
I absolutely agree with that. Where did I say otherwise?
But, I suggest that final escape to space occurs at higher and colder altitudes because the proportion of CO2 molecules participating must be less, not greater, when more CO2 is added, in order for equilibrium. The total number of CO2 molecules participating has to stay constant.
The problem with the SGA is that it stops too soon for the flaw to be seen. The crux of the SGA is where it refers to extra CO2 molecules absorbing photons at high altitudes. It neglects to say anything about the next, inevitable process, which is the following decay, with the “centralised energy packet” within the excited molecule distributing its energy into the various states available to it, in line with the Second Law of Thermodynamics. Must not forget that!
Thank you for your interest and for taking the trouble to respond.
Happy New Year to you, too.
PS to #159
Does anyone have the audacity to say they agree with my #147 +++
Can I take no replies as a “Yes”?
AEB
AEBanner, Proportion of CO2 has nothing to do with it. What matters is the number of CO2 molecules an IR photon is likely to encounter on it’s way to the exosphere. Each CO2 molecule adds to the probability of absorption and thence to backradiation and heating of the atmosphere.
The mistake you are making is that as you increase the ghg concentration, the atmosphere is no longer in thermal equilibrium. The IR that doesn’t escape is heating the atmosphere–and it does so until the temperature nudges up the blackbody curve and Energy IN=Energy Out once again. The IR photon does not care whether the CO2 molecule is in the troposphere or the stratosphere. To first order, the absorption cross section is the same. Yes, most of the energy transported to the mid troposphere got there via convection. That’s all the more reason why adding CO2 is effective in trapping the IR radiation emitted there (where you are above most of the water vapor). There is nothing magic about 280 ppm that says the greenhouse effect stops above this level. It doesn’t stop–that’s the whole point of the refutation discussed by Spencer and Ray Pierrehumbert.
You state that the total # of CO2 molecules participating has to stay constant. How do you get that? That makes no sense unless you assume that once a photon is absorbed that energy is out of play–patently false. As long as there is energy escaping in that band, it can be absorbed by adding CO2 to the atmosphere above where it was emitted.
I’m not sure what you mean by “cetralized energy packet”, but there’s no conflict with the 2nd law of Thermo. All that says is that if you dump energy into one mode, it will thermalize eventually.
I am still hung up on the saturation argument and the debate between AEBanner and Ray Ladbury has provoked me to try again for enlightenment.
1)The atmosphere is opaque to 13.5-17 micron IR emitted from the surface such that no photons of these wavelengths can pass to the upper atmosphere. Above the opaque layer, CO2 is present and able to absorb (and re-emit) photons of the appropriate wavelength. Where do they come from? They are clearly present at this level as can be determined from space (looking down). Presumably, they are produced from non radiative thermal energy convected from below reacting with CO2. Because of atmospheric opacity below, they can never get back to the surface but they can leave the atmosphere to space, albeit with increasing delay as CO2 concentration increases.
2) Suppose, for the sake of argument, that there were no molecules of CO2 above the opaque layer. How would the non radiative energy then leave the system? Presumably, slowly by diffusion resulting in significant warming in the high troposphere. If one were then to add a small amount of CO2, energy would escape more quickly in emitted photonic form. If this reasoning is correct, CO2 would cool the upper troposphere. However, there may come a point when adding even more CO2 would start warming it again. This suggests that both AEBanner and Ray Ladbury are both correct and that the outcome (warming or cooling) would depend upon the current CO2 concentration.
3)Suppose we have reached or are very close to a situation where the atmosphere at a given layer is opaque to all IR except that passing through the atmospheric window. Above that level, there is CO2 but almost no water vapour. Surface warming will lead to more water vapour but it won’t necessarily get any higher (vertically) in the atmosphere. Why, therefore, should there be a positive feedback? The second greenhouse blanket analogy won’t work in the case of water vapour.
4) How does energy above an IR saturated layer contribute to surface warming. It can’t be due to net downward re-emission of photons because of underlying opacity. Is it explained by downward convection of non radiative energy and, if so, is this effect predominantly at the poles?
5) Why try to explain warming on the basis of a second greenhouse blanket (high level CO2) when it is possibly more easily explained by a tighter wrapping of the first blanket (decreasing height from the ground as CO2 concentration rises)?
6) I have read differing interpretations relating to absorption and re-emission of photons. Are the emitted ones always of the same wavelength as those absorbed or only sometimes. If an excited CO2 molecule could lose some energy in collisions, could it then also emit a photon of lower energy than that absorbed which would presumably have a longer wavelength? If so, this would seriously constrain the blocking effects of CO2 at high altitude where there is minimal water vapour.
7) I have read that the brightness temperature measured from space for IR in the 15 micron band is 215K. If CO2 is blocking energy egress to space, this temperature should be falling. Is it?
It will be obvious that I am no physicist so can anyone help?
Reply to #161, Ray Ladbury
Thank you for your reply. I don’t think there is much difference between our two views of the overall Earth/atmosphere system, but there is a major difference in our thinking about one particular aspect. There have also been minor problems with understanding certain points of expression, which I shall come to later.
The Saturated Gassy Argument (SGA) would seem to be the thinking not only of Real Climate’s majority readers/contributors, but also the IPCC. Correct? This explanation of the Enhanced Greenhouse Gas Effect accepts that no additional anthropogenic warming can arise from the GHGs fairly close to the Earth’s surface, but that it occurs at high altitudes “where the air is very thin”. OK, no problem!
The basic SGA point is that a photon of the appropriate wavelength which is on its way out to space can be absorbed by a molecule of CO2, so reducing the output of energy to space which is required for energy balance to be maintained, and so increasing the temperature until balance is again achieved. Moreover, if this molecule relaxes again to a lower vib/rotational state by inter-molecular collision with another CO2 molecule or with one of oxygen or nitrogen, then its energy becomes kinetic energy of the atmosphere and thereby raises the temperature.
OK, so far, but that is not the complete story!
Now, let’s go back a bit, and think about that photon which was escaping to space, but was absorbed. I think we both probably agree that it originated in the troposphere. (This would be in line with Kiehl and Trenberth as mentioned in previous posts.) Therefore, its internal energy came from the kinetic energy of the troposphere, so making a cooling effect. When the photon is absorbed, its energy becomes the increased internal energy of the absorbing CO2 molecule. If then, the excited CO2 molecule relaxes by collision, as above, this amount of energy is put back into the troposphere, so making an equal heating effect, and restoring the original temperature.
Alternatively, instead of relaxing by collision, suppose the excited CO2 molecule spontaneously decays to its lower state by simply emitting a photon which would be of the same energy (?).
This new photon may be emitted upwards or downwards by the familiar argument. If it goes upwards, it can escape to space, simply replacing the original one. So energy balance is left undisturbed. If it goes downwards into the troposphere again, then it will be “thermalised” by collision, so restoring the energy removed from the troposphere to create the original photon. So there is no change in the temperature of the troposphere.
This argument applies whatever the concentration of carbon dioxide. If more is added, the same processes operate as above, and there is still no overall change of temperature. So there is no enhanced greenhouse gas effect.
Look at it another way. Carbon dioxide plays a part, as just described, in providing photons in the troposphere by the collision/excited molecule/photon emission process, and some of these photons escape to space to ensure energy balance. This balance will be achieved when a certain number of carbon dioxide molecules are participating in the process. (Of course, it does not have to be the self-same molecules all the time.)
So only a certain proportion of the total number of CO2 molecules in the whole atmosphere are needed to do the job, to maintain energy balance.!!!!!
If now, the amount of CO2 is doubled, then the required PROPORTION is halved, so the participating number is kept constant. This relationship applies in general; the participating proportion of CO2 molecules multiplied by the total number of molecules of CO2 must remain constant, and so no change in temperature occurs. This can be seen mathematically in my #147, in the equation kb = b1.
A couple of small points in your last reply.
I’m sorry, but I don’t know where you thought I had got hung up on 280 ppmv. I have explained my thoughts above on this matter without reference to any particular CO2 concentration. Again, sorry about the “centralised energy packet” phrase; I was just referring to the increased internal energy of the excited CO2 molecule.
I shall be very interested in replies to help my understanding of this problem. To sum up, I have no problem with the natural greenhouse gas effect, but I do not accept the current explanation for the Enhanced effect. I believe there is global warming, caused by some other, as yet unknown, process.
Re #162
The problem is that for every question you ask you are likely to get more than one reply. How do you tell which one is correct?
When you ask 7 questions then you have a real problem sorting out the answers, that is if anyone can be bothered to answer 7 questions knowing that if they do, then they will be contradicted by other replies :-(
Douglas Wise (162) — Looks to be that you want to read a good book on Atmospheric Physics.
AEBanner says: “This explanation of the Enhanced Greenhouse Gas Effect accepts that no additional anthropogenic warming can arise from the GHGs fairly close to the Earth’s surface, but that it occurs at high altitudes “where the air is very thin”.”
Actually, this is not totally correct. Increasing CO2 means that there are more CO2 atoms to absorb in the wings of the spectrum, so more warming occurs even at low altitudes.
AEBanner then says after an argument about energy balance: “This argument applies whatever the concentration of carbon dioxide. If more is added, the same processes operate as above, and there is still no overall change of temperature. So there is no enhanced greenhouse gas effect.”
Your reasoning in this argument is incorrect on several fronts. First, the upper atmosphere is cooler than the surface and lower atmosphere. There fore a ground-state CO2 molecule struck by another molecule is less likely to have its vibrational state excited than is an excited CO2 molecule to relax via collision. Therefore there are fewer CO2 molecules in excited states to emit IR photons–thus less energy is lost at this level. Second, there is a continual flux of IR from the surface and lower levels. Third, you cannot assume that if the photon is emitted upwards that it WILL escape. It has a probability of escaping, and that probability decreases as we pile more CO2 molecules in its way, does it not?
Finally, you keep clinging to equilibrium when the system is not in equilibrium. Yes, it starts out that way before we start heating the surface, but once IR photons start flowing from below, the cool gas in the upper atmosphere is NOT in equilibrium, but is being heated from below.
Re: your comments on my comments–280 ppmv is concentration that causes the “natural” greenhouse effect prior to the industrial age. And again, why should the effect magically stop at 280 ppmv?
Douglas Wise
1)”Because of atmospheric opacity below, they can never get back to the surface”–not strictly true. Each absorption is followed by relaxation either radiatively or collisionally. Via many steps, photon can reach Earth. Also, if it doesn’t reach Earth, it heats the atmsophere. Thermal energy can travel down. Also, a warmer atmosphere radiates more photons (down and up), so there is radiation from the atmosphere making it to the ground.
2)The energy cannot leave the system nonradiatively. If it diffuses up, it’s still in the system. Opaque is not really a description. Photons of the proper frequency originate not just at the surface, but wherever there is CO2–maybe even the molecule next door.
3)Again, remember GHGs radiate as well as absorbing. They radiate less than the surface because they are cooler.
4)Again, the problem is opacity–IR is emitted throughout the atmosphere and can be absorbed at any level. Heat the gas up–even just above the surface, it emits more IR, but at low levels it can also transport heat near the surface via convection.
5)Not really a second blanket–think of it as increasing the number of muggers a commuter has to get past on his way home.
6)The CO2 energy line is actually a range of energies–frequency vs intensity of emission looks like a bell curve, but with long wings to either side. Emission can occur anywhere within this band–collisions can distort the absorption or emission spectrum as well.
7)The blocking of energy by CO2 is THE REASON why the temperature in that band looks lower. It’s because less energy can escape from below. In effect, adding more CO2 raises the level where significant IR photons in that band can escape, and since higher=colder, the temperature of emission does decrease.
Don’t give up. I have a PhD in physics and 20 years experience (or you could look on it as I’ve had 20 years to forget what I learned), and this took work for me, too. Keep asking questions.
Reply to #166 Ray Ladbury
Again, thank you for taking the trouble to reply, but I am still unable to accept your reasoning. Things have become somewhat complicated, and I think it might make for better discussion if some simplification were attempted. [edited]
[Response: We won’t allow discussion threads to be hijacked with long, off-topic diatribes. You can continue this discussion off-site if you wish, but no more of this here. -mike]