Much research effort over the past years has gone into reconstructing the temperature history of the last millennium and beyond. The new IPCC report compiles a dozen reconstructions for the temperature of the Northern Hemisphere (including of course the original “hockey stick” reconstruction, despite opposite claims by the Wall Street Journal). Lack of data does not permit robust reconstructions for the Southern Hemisphere. Without exception, the reconstructions show that Northern Hemisphere temperatures are now higher than at any time during the past 1,000 years (Figure 1), confirming and strengthening the conclusions drawn in the previous IPCC report of 2001.
Fig. 1: Figure 6.10 (panel b) from the paleoclimate chapter of the current IPCC report (see there for details).
“Climate sceptics” do not like this and keep coming up with their own temperature histories. One of the weirdest has been circulated for years by German high-school teacher E.G. Beck (notorious for his equally weird CO2 curve). This history shows a medieval warm phase that is warmer than current climate by more than 1 ºC (see Figure 2). So how did Beck get this curve?

Fig. 2, modified from E.G. Beck (we added the green parts).
The curve is a fake in several respects. It originally is taken from the first IPCC report of 1990: a scan of the original is shown in Figure 3. At that time, no large-scale temperature reconstructions were available yet. To give an indication of past climate variability, the report showed Lamb’s Central England estimate. (Unfortunately this was not stated in the report – an oversight which shows that IPCC review procedures in the early days were not what they are now. We will post in more detail on the history of this curve another time.)
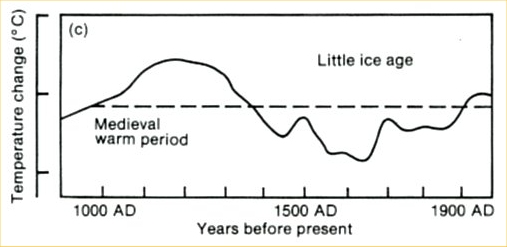
Fig. 3. The past millennium as shown in the first IPCC report of 1990, before quantitative large-scale reconstructions were available. This curve was based on Lamb’s estimated climate history for central England.
But Beck did not stop at simply using this outdated curve, he modified it as highlighted in green in Figure 2. First, he added a wrong temperature scale – the tick marks in the old IPCC report represent 1 ºC, so Beck’s claimed range of 5 ºC exaggerates the past temperature variations by more than a factor of three. Second, the original curve only goes up to the 1970’s. Since then, Northern Hemisphere temperatures have increased by about 0.6 ºC and those in central England even more – so whatever you take this curve for, if it were continued to present, the current temperature would be above the Medieval level, as in the proper reconstructions available today. As this would destroy his message, Beck applied another fakery: he extended the curve flat up to the year 2000, thereby denying the measured warming since the 1970s. With this trick, his curve looks as if it was warmer in Medieval times than now.
When approached directly about these issues, Beck published a modified curve on a website. He changed the temperature range from 5 ºC to 4.5 ºC – but he shortened the arrow as well, so this was just cosmetics. He also added instrumental temperatures for the 20th Century at the end – but with his wrong temperature scale, they are completely out of proportion. (In fact his version suggests temperatures have warmed by 2 ºC since 1900, more than twice of what is actually observed!)
Beck goes even further: in a recent article (in German), he has the audacity to claim that his manipulated curve is right and the more recent scientific results shown by IPCC are wrong. And for years, he has offered his curve on an internet site (biokurs.de) that distributes teaching materials for schools, with support from German school authorities. It is quite likely that his fake curve has been shown (and will continue to be shown) to many school children.

[[Barton Paul Levenson (#266) wrote:
Your 10 meter figure is completely bogus. For a concentration of 400 ppm, 99% of surface radiation is absorbed at 18 meters for the 1.9-2.1 micron band, at 82 meters for the 2.6-2.9 micron band, at 625 meters for the 4.1-4.5 micron band, and for the 13-17 micron band, where the CO2 greenhouse effect does most of its work, at 7,800 meters. Want the math?
…..
Is there a link or offline article? This sounds like interesting stuff – not that I expect to be able to follow it. But I guess there is always hope. ]]
Sure. The transmissivity of a column of air is:
exp(-k p L)
where k is the absorption coefficient, p the partial pressure, and L the path length. I’m using Essenhigh’s (2001) reductions for the absorption coefficients, which seem to be correct even though he applied them wrongly in his article. For k the units are reciprocal meter atmospheres (m-1 atm-1). Pressure (misnamed “concentration” in Essenhigh’s article) is in atmospheres, and path length L is in meters. The units all cancel, giving a dimensionless answer.
For CO2 the coefficients for the four major infrared bands (at 1 atmosphere pressure and 288 degrees K. temperature) are:
1.9 – 2.1 microns: 656
2.6 – 2.9 microns: 139.4
4.1 – 4.5 microns: 18.37
13 – 17 microns: 1.48
The absorptivity is simply 1 minus the transmissivity, since scattering is negligible for the infrared in Earth’s atmosphere. (It has to be taken into account for Venus’s atmosphere, though.)
For the path lengths, just substitute 0.99 for the absorptivity and solve for L.
Essenhigh’s article can be found at:
http://pubs.acs.org/subscribe/journals/ci/31/i11/html/11box.html
He makes the same mistake that DocMartyn and Barrett make — assuming that since most of the ground IR is absorbed close to the ground, adding more CO2 won’t have any effect.
Re #283 — yes, the lapse rate generally increases with altitude in the troposphere. It starts out at about 4.75 K/km near ground level and increases to almost 9.8 K/km near the tropopause. The average is around 6.5 K/km, which is what NASA/NOAA/USAF used for the 1976 Standard Atmosphere.
[[On Earth the clouds are formed from water, on Venus from sulphur dioxide, and on Mars from dust.]]
At Venus temperatures, sulfur dioxide is a gas. The Venus clouds are a 75%-85% aqueous solution of sulfuric acid (H2SO4.H2O). They are, like Earth clouds, drops of liquid.
The Mars clouds are water droplets and ice droplets, and also CO2 liquid and ice. Mars does have dust in its atmosphere much of the time, but generally at a much lower altitude than the clouds.
Re #299 Barton,
Writing that Barrett is a pseudoscientist is both incorrect and an ad hominen argument, so not worth discussing.
You wrote “For a concentration of 400 ppm, 99% of surface radiation is absorbed at 18 meters for the 1.9-2.1 micron band, at 82 meters for the 2.6-2.9 micron band, at 625 meters for the 4.1-4.5 micron band, and for the 13-17 micron band, where the CO2 greenhouse effect does most of its work, at 7,800 meters. Want the math?”
I think that 30 m makes a fair average of your figures, especially if water vapour is included. The point is that the absorbed radiation is not re-emitted because the excited molecules are relaxed by collisions with the other air molecules. The energy that would go to provide the energy for re-emission has gone to heat the air. It is really quite simple.
What is not so obvious is that increasing CO2 concentration will warm the surface, but it does by conduction. What is obvious is that radiation changes near the top of the troposphere cannot drive the surface temperature 10 km away!
Re #303
At the surface temperature of Venus sulphur evaporates and forms a gas which condenses and forms a cloud. The surface temperature of Venus is set by the vapour pressure of sulphur, just as the surface temperature of the Earth is set by the vapour pressure of water, roughly speaking. Mars heats up until there is a dust storm which cools it down. Planetary climates are dynamical systems where at times either positive or negative feedbacks dominate. Mostly the negative feedbacks dominate because they are stable, but short catastrophes due to positive feedbacks can happen.
“I know the extinction coefficient at peak is higher for cold molecules than for hot ones, I even stated it, but what happens at either side of the absorbance peak?
Take two gaussians representing emission and absorbance, give the cold absorption peak half the line width of the hot emission peak. Take one away from the other and you find that 32% of the emission gets through, even though 100% is absorbed at the peak. Its not complicated.”
GIGO. The most obvious effect of changing altitude is to change the number density of the CO2 molecules. This changes the total band absorption in a straight forward manner
There are two major effect of changing temperature. The first is to change the distribution of ground vibrational level quantum states, which changes the opacity of the system as a function of photon frequency. The second is to change the thermal distribution of population in the first excited level of the two degenerate bending modes, which means that the intensity and frequency distribution of the emission changes. Both of these are simple to calculate on a line by line basis that should be in the reach of a senior year physics or chemistry major.
In all cases up to well above the stratosphere, collisional energy transfer assures local thermodynamic equilibrium (equivalent to saying that the distribution among quantum states can be described by a temperature)
A second order effect is to change the pressure and temperature broadened line shapes of the individual ro-vibrational lines, which means that some extra light sneaks through colder, less dense air above warmer denser volumes. The study of line shapes is fascinating. They are not gaussian, but best described as a combination of gaussian and lorentzian with a very small continuum contribution.
Suffice it to say that people have spent lifetimes studying these things and they are well modeled. There is a government/commercial product called HiTRAN which allows one to calculate all these effects.
Alastair, is this your own theory? Do you have sources supporting the idea you base it on?
I go looking for support but what I find when I search is you saying this in many places, and others disagreeing, like this:
http://forums.edgcm.columbia.edu/showthread.php?t=649
but I haven’t found where you’re getting it from.
Re 279: Rod B., First, let me state that when I comes to climate science, I am not an expert. I know enough physics to follow most of the arguments up to a certain point, and I find that they make sense based on what I know. For the portions that are too technical for me to follow closely, I have relied on the summaries prepared both by climate scientists and by independent reviewers from outside the field–e.g. the National Academy of Sciences. What is more, I have had the opportunity to meet some climate researchers and discuss issues with them. Suffice to say that radical and rash are not adjectives that apply to them. My question is that given that the climate scientists are not stupid, and that the research they do has been reviewed to death by outside panels without the basic conclusions being significantly changed, and given that the predictions have erred, if at all, on the conservative side, wouldn’t it take a certain temerity on my part to assume that I could tell the community of climate scientists how to do the job that many of them have been doing for >20 years?
I think that what climate scientists find frustrating is the fact that for most of them their real objections arise from the economic or political costs of addressing climate change, while what they attack is the science–which they rarely understand. In my opinion, if one’s objections arise from economics, it would seem to be more profitable to focus one’s efforts toward ensuring that the remediation is economically sound. If from politics, it would seem that ensuring the remediation is narrowly focused and does not verge toward social engineering. Instead, most so-called skeptics attack the science–the easiest point to defend and the place where their offensive weapons are weakest. Not good strategy.
As to your technical objections, the assertions of scientific consensus have nothing to do with any type of vote. Scientific consensus has to do with the strength of evidence–specifically whether it is sufficiently strong to convince the vast majority of experts. The goal is not to convince ALL the experts, precisely because it is realized that some scientists are nuts. In fact any scientist is nuts when it comes to some issues. Einstein was implacably opposed to quantum theory. Should we have said, “Oh, well, don’t try making that laser or that transistor. It needs more study?”
Pressure broadening of spectral lines is well understood and has been measured in the lab. Why should it suspend itself in the atmosphere?
As to your qualms with the “accuracy”–do you deny that significant warming is occurring? Given the effects we are seeing, I would call the estimates of warming conservative if anything? And if you believe that we are seeing significant warming, the question becomes where the energy is coming from. Climate scientists have developed an answer to this question. It is based on known physical mechanisms. It is of sufficient size to explain the effect. The underlying cause is known to be present and is understood as well. Looking back, it also explains a lot about past warming epochs. Pretty much all the evidence is consistent with this mechanism–or is becoming so. There is no alternative explanation that comes close to explaining the mechanism. Models based on this hypothesis have shown good ability to predict effects like the eruption of Mt. Pinatubo. I would argue that by opposing good science when you have nothing better to offer and no concrete objections other than you own suspicion, you undermine your credibility.
Alastair, what are you talking about when you say, “What is obvious is that radiation changes near the top of the troposphere cannot drive the surface temperature 10 km away!”. This is a gross mispresentation of how these processes work.
Radiation changes at the top of the atmosphere are the result of processes integrated throughout the atmosphere… again, see the above links to the IPCC and to AIP in #290:
The issue here is all about …the flow of heat and radiation up and down through a vertical column that rose from the ground to the top of the atmosphere. You started with the radiation, tracking light and heat rays layer by layer as gas molecules scattered or absorbed them. This was the problem of “radiative transfer,” an elegant and difficult branch of theoretical physics. (from Weart, AIP)
There are a number of ways an excited-state molecule or atom can relax to the ground level (photosynthesis is an area where such concepts are very important).
At low temperature and pressure, there is less opportunity for molecules to collide with one another (think top of the atmosphere). At higher pressures and temperatures, the molecules are more likely to interact, which leads to a broadening of the absorption lines. This is the reason that if you wander into an experimental spectroscopy lab, you will usually find tanks of liquid nitrogen and or helium for immersing samples in – spectroscopists use this effect to get sharper, more discrete spectra (from which more information can be extracted).
So, let’s review Basic Radiation Math (Weart) again:
As for CO2 itself, the old measurements made at sea-level pressure had little to say about the frigid and rarified air in the upper reaches of the atmosphere, where most of the infrared absorption takes place. In the early 1950s precision measurements at low pressure, backed up by lengthy computations, showed that adding more CO2 really would change how the atmosphere absorbed radiation. While the total absorption might not change greatly, the main site of absorption would shift to higher, thinner layers. And as Callendar had explained, shifting the “screen” in the atmosphere higher would mean more radiation going back down to warm the surface.
Here is a figure which reveals the effect for oxygen at the 0, 3, 10 and 20 km levels in the atmosphere. You can see the broad, smooth absorption at sea level, and the ‘picket fence’ effect at high altitudes. The same ‘pressure-temperature band broadening effect’ applies to CO2 (the rate of molecular collisions depends on both temp and pressure). Adding CO2 to the upper atmospheric levels results in increased absorption as a result – one could say that the picket fence is being raised.
One interesting result of all this absorption and emission is that as the troposphere warms due to greenhouse gases, the stratosphere cools. That doesn’t seem to be the only reason the stratosphere is cooling; ozone destruction is also involved. See Why does the stratosphere cool? The stratospheric cooling rules out increases in solar radiation as the cause of global warming. (Keep in mind that the stratosphere is where temperature increases with height – but at low pressure – thus CO2 in the stratosphere helps cool the atmosphere via infrared emission to space. O2 and N2, the main components, don’t have much IR emission – see the figure in the above link).
The basics of these phenomena seem to have been understood some 50 years ago. So why are they brought up over and over again by skeptics and contrarians? Is it all just to create the false appearance of controversy and scientific disagreement?
As far as ‘ad hominem’ arguments (‘against the person’) – well, if a person repeatedly ignores factual evidence, distorts basic scientific facts and makes false claims, over and over again, even after being pointed repeatedly to the actual facts of the matter, then they lose credibility. The first rule in science is that you have to be willing to admit error – otherwise, you’re just pushing some position – most likely for political or economic reasons.
302 Barton, I believe that the standard Atmosphere at 80 N calls for a lower lapse rate at the higher levels of the troposphere. A key here is to look for real time effects of CO2, ideally this is done in prolonged darkness.
304 Alastair, I do know if a segment of the Upper Atmosphere is changed the rest follows, all levels affect each other hydrostatically. A warming in the Upper Atmosphere affects the lower atmosphere. By the Way, many news releases have shown that models underestimated current warming, they were wrong in intensity of the warming but not on the trend.
Re# 304
Please please please pick up a textbook on radiative transfer……when I have some time I can even recommend some to you.
PS. The higher the temperature the more emission. Google Kirkof’s law it is quite valid for altitudes below about 100km or so. Most of the lab work to verifiy this is decades old. However, Eli Rabbet some days ago gave a good reference even to some nice lab work (Evens and ?) demonstrating very very good agreement between IR radiative transfer calculations and lab observations. Not to mention tonnes of surface radiation balance observations and closure studies (google baseline surface radiation network (BSRN) )
Actually Astair forget the BSRN it is not as informative as I thought it was….was thinking of some other siye but can not think of it right now.
Anyways…have you ever considered that if IR rad transfer worked like you think it does then how could the atmosphere emitt IR radiation to space at all ? In fact what would stop the temperature of the whole system from ever increasing ?
Re #312
Hi David,
Some radiation is emitted to space from the surface through the “window”, and CO2 emits radiation at a blackbody temperature of around 220K, but you are right. The surface temperature would go through the roof. This is what has happened on Venus. It stopped rising when the surface melted and the sulphur dioxide clouds formed. The Earth is similar, only the clouds of water are formed by a much lower surface temperature than that on Venus.
Everyone thinks that the climate is driven by a nice simple negative feedback system, but it is not. It can also be chaotic.
Re #311
I read lots of books on radiative transfer and they are all wrong, except the “Handbook of Atmospheric Science” by Hewitt and Jackson, 2003. They write “Clearly the temperature in the tropsophere does not vary in this way.” p. 44.
Kirchhoff’s Law is not valid for the 30 m of the atmosphere nearest the surface. There the radiation is absorbed and heats the air. It is not re-emitted. Compared with the 100 km you mention 30 m is very insignificant but that is where greenhouse effect works. And that is where we live!
Re #310
Wayne,
Changes in the pressure of the upper atmosphere can affect the lower surface, but changes in radiation that is absorbed by greenhouse gases are only transmitted 30 m, and can no way reach 10 km down to the surface.
Re #309
Ike, You quote Weart As for CO2 itself, the old measurements made at sea-level pressure had little to say about the frigid and rarified air in the upper reaches of the atmosphere, where most of the infrared absorption takes place.
But most of the infrared absorption happens at the base of the atmosphere. Just think about the Beer-Lambert Law. it is only solar radiation that is absorbed at the top of the atmosphere.
Re #307
Hi Hank,
It would be wrong for me to claim that it is all my own thinking since it was Jack Barrett’s paper that gave me the clue. Heinz Hug has also supported Barrett. You will find some of their ideas on John Daly’s site ie http://www.john-daly.com/forcing/hug-barrett.htm . Of course all three of them are out and out sceptics, but I was puzzled how Jack Barrett got a paper published in the peer reviewed journal Spectrochimica Acta 51A, 415-417 (1995). The reason was he had a point. I was already suspicious of the models because they cannot reproduce abrupt climate change. The outgoing radiation scheme is paramaterised and does not use physical laws unlike most of the rest of the code in the GCMs. Therefore, that seemed the most suspect area. It is only now, after five years of research, that I really understand how it is wrong, and how wrong it is!
Here is a link to a short paper I wrote a couple of years ago, with a few references, but never got published. http://www.abmcdonald.freeserve.co.uk/brief/brief.htm
Re #310 again.
Wayne wrote “By the Way, many news releases have shown that models underestimated current warming, they were wrong in intensity of the warming but not on the trend.” What I am saying is that with my model the greenhouse effect increases in proportion to the CO2, whereas the standard predict that the greenhouse effect will only increase with the logarithm of the CO2 concentration. What you wrote supports my theory – that global warming will be worse than predicted.
Re #311 again
I replied to Eli regarding Evans and Puckrin in post #285. Basically, they don’t address the real issue which is that the bottom 30 m of the atmosphere are not in thermodynamic equilibrium so Kirchhoff’s Law does not apply there.
# 290 Compare and contrast:-
“At the TOA, the net SW radiation is everywhere partially compensated by outgoing LW radiation (i.e., infrared emissions) emanating from the surface and the atmosphere. Globally and annually averaged, this compensation is nearly exact. The pattern of LW radiation emitted by earth to space depends most critically on atmospheric temperature, humidity, clouds and surface temperature. With a few exceptions, the models can simulate the observed zonal mean of the annual mean outgoing LW within 10 W m-2 (an error of around 5%; see Supplementary Material, Figure S8.7).”
“With a few exceptions”, how droll, no one can accuse the author of not having a sense of humour.
So the models do +/- 10 w m-2 do they.
Hansen, J.E., 2003. The global warming time bomb? Natural Science
“Climate sensitivity is the response to a specified forcing, after climate has had time to reach a new equilibrium, including effects of fast feedbacks. A common measure of climate sensitivity is the global warming caused by a doubling in atmospheric CO2 concentration. Climate models suggest that doubled CO2 would cause 3 °C global warming, with an uncertainty of at least 50%. Doubled CO2 is a forcing of about 4 W/m2, implying that global climate sensitivity is about 3/4 °C per W/m2 of forcing.”
http://www.naturalscience.com/ns/articles/01-16/ns_jeh.html
Doubling is 4.4 w m-2.
Now in most studies the fact that the models resolution is twice the reported signal would mean that the model was classified as “crap”. But I have been led to believe that this is not the case in this field; would these models be classified as “robust”?
———————————————————————–
#309
I note more flim-flam
“Here is a figure which reveals the effect for oxygen at the 0, 3, 10 and 20 km levels in the atmosphere. You can see the broad, smooth absorption at sea level, and the ‘picket fence’ effect at high altitudes. The same ‘pressure-temperature band broadening effect’ applies to CO2 (the rate of molecular collisions depends on both temp and pressure). Adding CO2 to the upper atmospheric levels results in increased absorption as a result – one could say that the picket fence is being raised.”
What you figure shows is that more of the emission spectra of O2 at low altitude can sneak through the gaps between the peaks at high altitude.
Take one line away from the other and you will get another set of picket fences that poke out into space.
—————————————————————–
can someone please tell me if the 14C02 pixie ate up the atmospheric 14Co2 generated by the H-bomb tests, or can someone tell me who it had a half-residence time of a decade while the rest of the CO2 has a half-residence time of 100 years. I am so dumb that I don’t understand how different isotopes of 14C02 and 12Co2 can have different sinks.
—————————————————————–
If the high atmosphere responds to increased levels of CO2 by heating the ground level. Why has the cooling and heating of the South pole been unchanged in the past 50 years, even though CO2 has risen by 30%.
Again, I am so dumb that I don’t know how the CO2 molecules in the atmosphere know that the antartic platau is higher than the majority of the Earths surface and decide not to send IR radiation here. Can someone tell me the mechanism by which the CO2 knows where on Earths surface it should radiate?
There is a lot of complex and contradictory information presented here on how the greenhouse effect actually works. I would like to step back and describe three different models that encapsulate some of these ideas.
1)The Blanket Model:
Greenhouse gases are like a blanket that trap radiation. There seems to be agreement that water vapor at the bottom 30 m of the atmosphere absorbs most of the longwave radiation from the surface. This models says that is where most of the greenhouse effect takes place. As the amount of carbon dioxide in this layer is low, the implication is that increasing carbon dioxide will make little difference.
Question: Is there any validity to the idea that when a greenhouse gas molecule absorbs radiation, this induces vibration which transfers motion, thus heat, to the surrounding atmosphere?
2) The Reflector:
This model recognizes that when a greenhouse gas absorbs radiation, it re-radiates it in all directions, including up and down. Greenhouse warming is caused by the portion of longwave radiation that is returned to the surface. Adding more greenhouse gas reduces the amount of radiation that is prevented from leaving the Earth and is instead returned to the surface. More of the greenhouse effect takes place higher in the atmosphere where the ratio of carbon dioxide to water vapor is higher, implying that carbon dioxide is a relatively more important greenhouse gas than in the first model.
Question (a) Eli Rabett in #306 talks about the effect of temperature (thus altitude) on emission of radiation from a greenhouse gas. If I understand correctly, this is due to a change in the vibration and rotation states that can occur in the molecule. But how does this affect the intensity of the radiation? Eli also mentions that atmospheric pressure broadens the absorption spectrum. Can this effect under Earth conditions be quantified, ie. is it relevant? I know it is important on Venus.
Question (b) Can the downwelling longwave radiation be measured at the surface? Can we tell from the wavelength which greenhouse gas it came from? Roger Pielke Sr. has a page that appears to do this, using a model rather than measurements. I don’t know if the data reported is accurate, but I think the miniscule effect of increasing carbon dioxide it suggests is not correct.
3) Radiation Balance
The radiation balance model begins with the observation that the temperature of the Earth is determined by the balance between incoming and outgoing radiation. The rate of outgoing radiation is determined by the temperature of the surface. Greenhouse gases effectively raise the radiating surface into the upper atmosphere, which is cooler, so less energy is lost and the Earth warms.
According to this model, the only greenhouse gases that matter are the ones that radiate into space, which are high in the atmosphere. As carbon dioxide is relatively larger compared to water vapor at these altitudes, it is a significant greenhouse gas.
Question: Is it correct that a greenhouse gas molecule radiates less energy when at a lower temperature? I am not sure what Eli means in #291 by “the absorption per molecule at line center is HIGHER for colder molecules.”
#319
“The rate of outgoing radiation is determined by the temperature of the surface.”
Not true, the rate of outgoing radiation may be proportional to the temperature or it may not at all; it depends on the water vapor pressure, temperature and atmospheric pressure. It is very easy for the atmosphere to unload a lot of heat, with little effect on temperature or to gain a lot of heat with very little gain of temperature. You can heat water/land on a windy day and the temperature remains low, as water evaporates. During the night, at the dew point you can dump alot of heat into space, without increasing the temperature (the temperature can even drop0 as you convert water vapor to liquid water, dropping the pressure.
Water is the key to the relationshop between heat input and temperature, if there is no water you have a high day time temperature and a low night time temperature, hence deserts are hot as hell in the day and cold enough to make icecream at night. In the middle of an equitorial ocean,the day/night swing is less than about anywhere else on the plant.
When your body wishs to loss heat, and maintainits temperature you sweat, excreting salt water (rich in carbonate) on to your surface.
“Kirchhoff’s Law is not valid for the 30 m of the atmosphere nearest the surface. There the radiation is absorbed and heats the air. It is not re-emitted. ”
Are you saying that the greenhouse gases stop to radiate close to the ground? Any reason for that?
“Here is a link to a short paper I wrote a couple of years ago, with a few references, but never got published. http://www.abmcdonald.freeserve.co.uk/brief/brief.htm”
Are you suggesting that the air close to the ground only are heated by radiation? Ignoring the explaination in the excellent McIlveen book you refered to that the air close to the ground is heated by conduction and micro convection. I dont think he mentions any significant heating from radiation close to the ground or have I missed that?
Note: Higher pressure==> more collisions ==> Local Thermodynamic Equilibrium. ==> Kirkoff’s law holds. It is only when the collision rate is low can the radiation field temperature and the kinetic temperature of the gas be different.
Section 2.2.2 Breakdown of thermodynamic equilibrium
In Atmospheric Radiation. Theoretical basis, 2nd Ed. R.M. Goody and Y.L. Yung, Oxford University Press, New York, ISBN 0-19-510291-6 (Pbk.)
If you can show what is wrong with this text book then there may be a Nobel waiting for you !
Alastair, I think that you need to be more precise in your language. To say that the molecules do not re-emit in the lower 30 meters is absurd. If the density of radiation were sufficiently high, they might absorb another photon very quickly, but for that to be true, you would have to have a population inversion, and I don’t think we have much stimulated emission going on. If a molecule absorbs a photon, it enters an excited state, which will have a finite lifetime. If the excited state is vibrational, it is possible that the molecule will relax by mechanical processes–e.g. imparting its extra energy to a neighboring molecule. It is true that this will be more likely where densities are high–e.g. in the lower atmosphere. However, there is bound to be some re-emission, and that re-emission will be isotropic. Moreover, it’s absurd to say only sunlight is absorbed at the top of the atmosphere. Atoms don’t care where they are. The language you are using makes it sound like you don’t understand atomic physics–which I don’t believe for a minute.
Re #319 Where are you getting your temperature figures for the South Pole from?
Here ?
http://www.antarcticconnection.com/antarctic/weather/hist_wxdata/southpole_station.shtml
which gives an annual average temperature of -48C over the last 32 year
or from here?
http://www.coolantarctica.com/Antarctica%20fact%20file/antarctica%20environment/climate_graph/vostok_south_pole_mcmurdo.htm
which gives an annual average temperature of -49.4C from 1955 to 1988.
In other words the temperature at the South Pole has risen by 1.4C in the last 20 years!
Re #322 I am not saying that the greenhouse gases stop radiating. In fact they continue to radiated just as they always do. What I am saying is that they absorb more radiation than they emit. This is because the radiation from the surface of the Earth is more intense than the radiation emitted by the greenhouse gases.
I am not saying that the air near the ground is only heated by radiation. Conduction will also play a part. On page 250 of the 1992 edition “Fundamentals of weather and climate” he writes “Consider a package of radiant energy emitted from the Earth’s surface in these heavily absorbed wavelengths [greenhouse gas bands]. It will be completely absorbed by the first 30 m of air, warming first the molecules of water vapour and carbon dioxide, and then almost immediately sharing this heat with the surrounding air molecules.” However, he continues “But by Kirchhoff’s law, water vapour and carbon dioxide must emit these same wavelengths with the same efficiency as they absorb them; …” This defies the law of conservation of energy!
I am saying that in the lower 30 m of the atmosphere Kirchhoff’s law does not apply because the system is not in thermodynamic equilibrium since the air temperature is changing.
All I can say is that no doubt you will find it difficult to accept that Robin McIlveen, Sir John Houghton, Keith Shine, Gavin Schmidt and Ray Pierrehumbert have all got it wrong. But as far as I can see that is the case.
[[I think that 30 m makes a fair average of your figures, especially if water vapour is included.]]
Not even close. Most thermal radiation from the Earth’s surface, and from layers of atmosphere, is at greater than 4 microns. The peak from Earth’s surface is at 10-11 microns.
[[ The point is that the absorbed radiation is not re-emitted because the excited molecules are relaxed by collisions with the other air molecules. The energy that would go to provide the energy for re-emission has gone to heat the air. It is really quite simple. ]]
It’s simple and irrelevant. The heated air then radiates more than it did before it was heated, and some of that extra radiation goes back down to the ground. Collisional de-excitation does nothing to prevent the greenhouse effect from happening. In fact, since collisional de-excitation happens much more often than energy loss from a CO2 molecule by radiation, most of the greenhouse effect actually works that way. Either way, radiation is absorbed, radiation is given off, and energy is conserved. The arguments of Barrett and Hug (and John Daly, whose site they appeared on) that collisional de-excitation somehow obviates the greenhouse effect is pseudoscience. It’s wrong.
[[What is not so obvious is that increasing CO2 concentration will warm the surface, but it does by conduction. What is obvious is that radiation changes near the top of the troposphere cannot drive the surface temperature 10 km away!]]
Conduction plays a very minor role in vertical heat transport in a planetary atmosphere. And changes at the tropopause do get all the way down to the surface, because the tropopause affects the level just under it, which affects the level just under that, etc. In fact, on Venus, a lot of the reason the surface is so hot is that the clouds absorb infrared so well, and the clouds are almost entirely confined to the top 5% by mass of the atmosphere.
[[Changes in the pressure of the upper atmosphere can affect the lower surface, but changes in radiation that is absorbed by greenhouse gases are only transmitted 30 m, and can no way reach 10 km down to the surface. ]]
This is just wrong. Changes in radiation at the top level affect the layer under that, which affects the layer under that, etc. Radiation doesn’t have to go all the way from the top to the bottom to affect the bottom. You can see this with a simple multiple-level slab model. Each layer gets radiation only from the immediately adjacent layers, but the more layers you add, the hotter the surface temperature. There is a law of diminishing returns involved, but the effect of one more layer stays significant for a very long time. That’s why Venus modeled with 100 blackbody layers is hotter than with 99. (Try it and see!)
[[There seems to be agreement that water vapor at the bottom 30 m of the atmosphere absorbs most of the longwave radiation from the surface. ]]
There is NOT agreement on that point! Alastair has repeated it several times, but I don’t know anyone aside from perhaps DocMartyn who would agree with him. As I demonstrated, radiation at Earth’s thermal peak is not 99% absorbed until it has gone almost 8 km. That’s for carbon dioxide. For water vapor, the absorption coefficient in the 12-25 micron range is 0.69 m-1 atm-1, so for a typical water vapor concentration of 0.4%, the transmission path length is 1.7 kilometers. Not 30 meters.
Re #323 One reason I have not shouted about this more before is that I was worried about someone else grabbing my Nobel Prize!
David, You wrote “It is only when the collision rate is low can the radiation field temperature and the kinetic temperature of the gas be different.” That is what Goody and Yung believe, but it is not correct. If the radiation field is being produced by the surface of the Earth, then it need not be in balance with the kinetic temperature of the air. In fact since the air is heated by the surface its temperature always lags that of the surface.
The easiest way to see this is that there is a region at the top of the atmosphere where radiation out exceed collisions, and at the base of the atmosphere there is a region where radiation in exceeds collisions. So there are two regions of non-LTE, with LTE in between.
Blair Dowden (#320) wrote:
It gets complicated, and obviously this is an area where I wouldn’t even consider myself an informed layman at this point.
But with that much said, I have picked up a little and looked around a bit. They talk about absorbtion and re-emission taking place within 30 m of the surface. This is true of the radiation which the surface is receiving directly from the atmosphere. However, there is absorbtion and re-emission between the atmosphere and itself, and this will affect the surface,albeit indirectly. As a result, your “blanket” model and your “reflector” model would be two different aspects of the same thing.
With regard to how the vibration and rotation of greenhouse gases are concerned, these determine the wavelength of the radiation itself, but not the intensity. The intensity of the effect is instead determined by how many of the molecules are excited and engage in re-emission (per second, if you wish). The reason is that the excited state itself is quantized, such that the molecules are able to absorb and re-emit radiation only within bands corresponding to the excited state itself.
But then there is also the blurring of the spectra at higher pressures. This is due to the fact that these molecules which are absorbing and re-emitting radiation are in motion as a result of their temperatures, colliding and either losing some amount of energy or gaining some amount of energy prior to absorbtion or re-emission – and as such more or less energy will be required to enter either the excited or grounded state.
While energy is quantized as the result of the uncertainty principle, such as in the case of electron orbitals surrounding the nucleus of an atom due to the uncertainty principle being applied to momentum and position, the uncertainty principle can also be expressed in terms of energy and time, and thus how much energy is lost or gained as the result of such collisions will come into play within the context of absorbtion and re-emission. Or so I would gather.
Now with respect to measuring either downwelling or upwelling radiation, yes, we can most certainly do this. There are a fair number of graphs off the web which show the spectra as measured at certain times of day, altitudes and locations. Some of the graphs are the result of models and some are the actual empirical results. The text should say. Moreover, we are able to distinguish the effects of carbon dioxide, water vapour, etc by where in the spectrum they make their contribution.
Now Pielke is right – about carbon dioxide making a very small contribution near the surface. Doubling it near the surface raises the temperature of the surface and the atmosphere by only a small fraction of one percent. The reason is that there is a great deal more water vapour near the surface than carbon dioxide.
However, he is worse than wrong when suggesting that carbon dioxide is itself negligible in its contribution to the greenhouse effect. This is because while carbon dioxide contributes directly contributes very little to the greenhouse effect near the surface, it contributes a substantial fraction (about one additional degree Celsius when doubled) at higher altitudes in the stratosphere where water vapour is either absent or exists in very small quantities.
Additionally, there is the indirect effect of carbon dioxide on the amount of water vapour in the troposphere. Doubling the carbon dioxide will raise the level of water vapour by a certain amount as the result of warming the surface, then the water vapour will contribute to the greenhouse effect, raising the temperature even more, but a smaller amount each time – in what is essentially a geometric sum until the initial doubling of the carbon dioxide indirectly raises the temperature not one degree but three degrees as the result of water vapour positive feedback.
Now with regard to radiation balance, this principle applies at the surface and at each level in the atmosphere. The amount of radiation entering must equal the amount of radiation coming in if any part of the system is in equilibrium.
But it applies in both directions – such that downwelling radiation being re-emitted by carbon dioxide in the stratosphere is able to have a substantial effect at the surface. And both upwelling and downwelling radiation are important. Upwelling radiation leaving the surface may be absorbed and re-emitted as downwelling radiation in one or more steps and at different altitudes. If this were not the case there would be no greenhouse effect.
Now it is quite possible that I didn’t answer all of the questions you posed. It is also possible that I got one or more things wrong. If so, hopefully someone will correct this. But I believe I was able to answer most of your questions and got most of it right. In any case, the answer is principally that each of the “models” you mentioned are true, but in one way or another, they are describing different aspects of the same thing.
re 330. “One reason I have not shouted about this more before is that I was worried about someone else grabbing my Nobel Prize!”
Start small. Publish your results/theory. I am sure we all eagerly await your peer-reviewed paper in various physics and atmospheric scientific journals. While the literally thousands of physicists and atmospheric scientists around the world for past decades are all wrong and you are correct. ;-)
PS
There is one more point which deserves emphasis in my response to Blair Dowden (#320): the reason why we focus on carbon dioxide rather than water vapour has to do with their relative lifetimes in the atmosphere. Water vapour will fall out very quickly as the result of precipitation but carbon dioxide will remain in the atmosphere for a very long time. The balance of water vapour maintains itself through precipitation and evaporation. As such, any increase or deficit in water vapour will be largely irrelevant if it is not due to the increase or deficit in some greenhouse gas with a longer lifespan – such as carbon dioxide, as carbon dioxide is the second most prevailent greenhouse gas and has a long lifespan, it should be our primary focus – not water vapour. It is the primary “throttle” which determines the behavior of the system.
I hope this helps.
Alastair, you are panicking over boundary problems and getting wrapped aroung the axle over linguistic trivialities.
First, very little of the atmosphere is at the boundary with Earth’s surface, and less of it is at the “boundary” with space. The atmosphere IS more or less in equilibrium with the radiation field because the ghg interact strongly with the outgoing radiation from Earth’s surface. Yes, collisional relaxation is important, but if a molecule can relax due to collisions, it can also be excited by them and then decay via emission of a photon. This MUST happen, as otherwise there is no way for energy to get out of the system and temperature would heat up indefinitely–an effect I have not noticed happening. What happens is this: Start with a cool atmosphere and a warm surface. Outbound IR photons are absorbed by the ghg. Some re-radiate, but others are relaxed collisionally until the near surface region is roughly isothermal with the surface. The relatively larger number of ghg molecules in their excited states will radiate more IR photons in that bandwidth, and the atmosphere is effectively net transparent. As you move upward, the atmospheric density and IR photon density both decrease, the mean free path of an IR photon increases and the probability of absorption decreases, but is still finite. Alastair, here’s a hint. The guys who write text books are pretty smart and have generally been doing research in the subject for a lot of years. If you are finding that your answers don’t agree with them and their answers all pretty much agree with each other, who do you think is more likely to be wrong?
Re “30 meter infrared absorbtion”: It seems that it would be almost trivially easy to test this. Set up an infrared source and detector 30 meters apart, see if the detector registers the source.
I’m not certain from what’s been posted whether you’re saying that all infrared is absorbed, or just particular bands. If it’s all, then the claim is easily disproved by e.g. infrared night vision devices. If major parts of the IR spectrum were absorbed in that short a distance, they’d be as useful as binoculars in a fog bank.
Re #324: Ray, you said “If a molecule absorbs a photon, it enters an excited state, which will have a finite lifetime. If the excited state is vibrational, it is possible that the molecule will relax by mechanical processes–e.g. imparting its extra energy to a neighboring molecule.” Can you tell me relatively how much energy is lost to neighboring molecules, compared to that which is re-emitted, for a water vapor molecule at sea level? I am trying to understand if this process is a significant part of the greenhouse effect. Because most of the neighboring molecules are not greenhouse gases, I would think that there would be very little re-emission from them.
Re #327: Barton, you said “since collisional de-excitation happens much more often than energy loss from a CO2 molecule by radiation, most of the greenhouse effect actually works that way.” This contradicts what I understood (or maybe mis-understood) from Ray Pierrehumbert. Does this mean that the altitude at which a greenhouse gas molecule radiates into space is relatively unimportant?
Re #329: Barton, sorry for repeating misinformation. Do you have a reference to a table of greenhouse gas absorption coefficients?
> If major parts of the IR spectrum were absorbed …
http://www.weather.gov/sat_tab.php?image=ir
http://coolcosmos.ipac.caltech.edu/cosmic_classroom/ir_tutorial/irwindows.html
#328 Barton, layers indeed, some call them Gravity waves, in the small industry refraction business they are called ducts, micro layers, visible through very thick atmospheres at sunset, some have steep lapse rates causing Jules Vernes green flashes. How layers are stacked? What thickness? just what is a natural density layer? Cutting edge subjects.
Alastair has one strong point, amongst others, Polar temperature models are off by 25 years, may be because we don’t quite mimic these layers yet (polar Ocean ice and air are greatly intertwined), I propose any modeller to present Polar projected upper air profiles, especially those of 25 years from now, may be they match current sun disk dimensions. However, whatever it is, something unfortunately is wrong, and it would be good to find out about it real fast.
Aren’t we into deja vu all over again by now?
Search for the kinds of questions being asked in this thread — they’re repeated.
E.g.
https://www.realclimate.org/index.php?p=193#comment-5233
https://www.realclimate.org/index.php?p=193#comment-5251
Re: 329 “For water vapor, the absorption coefficient in the 12-25 micron range is 0.69 m-1 atm-1, so for a typical water vapor concentration of 0.4%, the transmission path length is 1.7 kilometers.”
Is this ln I/Io = – abc with I/Io = .01, a= 0.69 m-1 atm-1, b = 1.7 km, and c = 0.004 atm?
Well, Hank, the questions are repeated, but clear answers are still not forthcoming. I keep hoping.
Re #333
Can I just say to Tim and Blair that the reason I am concentrating on CO2 is purely because the way it behaves is a lot simpler than H2O, which is far too complicated for a blog. Unlike CO2, H2O varies in concentration from place to place. Moreover it does radiate in continuum mode (ie as a blackbody) as well as lines, some of which interfere with the CO2 lines. It also contains latent heat, and so water vapour carries energy high into the atmosphere, well beyond the limits of even some of the weaker lines that are absorbed by CO2 and H2O.
Moreover, when water vapour condenses, it forms clouds which radiate blackbody radiation both back to the surface enhancing the greenhouse effect, and out to space from a level of the atmosphere where there is little water vapour above it which can absorb, and so helps to cool the atmosphere. Thus each cloud both warms and cools by radiating. This is possibly a simplification too far, since it is generally accepted that high clouds cool the atmosphere and low clouds warm the surface.
Obviously, it follows that water is much more important greenhouse player than CO2, but to explain where the models are handling the outgoing long wave radiation incorrectly, it is easier to talk about an atmosphere where CO2 is the only greenhouse mechanism, and this is what I may have implicitly and explicitly done.
The idea that the persistence of water vapour in the atmosphere somehow affects its potency is a myth that is popular amongst anti-sceptics keen to explain why CO2 is more important than water vapour, despite water vapour playing a greater role. The real answer is that the small effect from CO2 is enough to keep most of the Earth’s surface above freezing point. Remove all the CO2 from the atmosphere and the surface of the Earth would cool, removing pretty well all the water vapour from the atmosphere. Without any greenhouse gases the surface would freze over. This is what may have happened 600 Ma ago when the last Snowball Earth occurred. http://en.wikipedia.org/wiki/Snowball_Earth
I have heard reference to the cooling both globally and in the Northern hemisphere following the eruption of Pinataubo. Now I know that this placed vast amounts of particulates and sulphur oxides into the atmosphere, cutting the Earths energy input.
I have been looking and can find no statistically valid drop in temperature, either world wide or locally. Given that many people quote temperature changes following the eruption as validating their modelling approach, does anyone have the data to back up this claim?
DocMartyn and Alastair,
Your comments are disingenuous and seem aimed at creating nothing but false controversy. There are so many errors in you understanding of basic physical processes that it’s pointless to even respond to them more than a few times – such as:
Alastair: “But most of the infrared absorption happens at the base of the atmosphere. Just think about the Beer-Lambert Law. it is only solar radiation that is absorbed at the top of the atmosphere.”
DM: “Have heard reference to the cooling both globally and in the Northern hemisphere following the eruption of Pinataubo… I have been looking and can find no statistically valid drop in temperature, either world wide or locally.”
Alastair: “What I am saying is that they [greenhouse gases] absorb more radiation than they emit. This is because the radiation from the surface of the Earth is more intense than the radiation emitted by the greenhouse gases.”
Anyone who has been reading this site for any period of time knows that these arguments are nonsense. This is just a repeat of previous contrarian efforts to create ‘scientific controversy’ and attack the scientific credentials of the authors of this site. Keep in mind that just because someone says “I’m on your side” doesn’t mean it’s so!
Radiative transfer physics sure is complicated, isn’t it? Nevertheless, it’s been fairly well understood for 50 years or so – unless Alastair is the new Einstein. There’s not much in the publication record, however…
DocMartyn (#343) wrote:
I would suggest the following technical article and the peer-reviewed papers it references:
Study of the effects of the pinatubo volcanic eruption using the UIUC stratosphere/troposphere GCM with interactive photochemistry
E. Rozanov, M. Schlesinger, F. Yang, S. Malyshev, N. Andronova, V. Zubov, and T. Egorova
http://www.aero.jussieu.fr/~sparc/SPARC2000_new/PosterSess3/Session3_3/Rozanov/P_3_3_13/Pinatubo.html
Re: Alastair McDonald (#342)
Thank you for going into some detail on the various effects of water vapour. Given the points I was trying to make in #331 and the postscript #333 regarding carbon dioxide, it seemed that going into the effects of water vapour in greater detail would have been at best a distraction. However, treating it separately as you have just done is a valuable contribution.
One point though – I do not believe that the effects of water vapour are so complex that the essentials cannot be dealt with by the contributors in one or a few essays. Indeed, your concise post would seem to suggest as much.
[[Re #329: Barton, sorry for repeating misinformation. Do you have a reference to a table of greenhouse gas absorption coefficients? ]]
Yes, here it is:
http://pubs.acs.org/subscribe/journals/ci/31/i11/html/11box.html
I’ve done some unit manipulation on his figures so I can work in absorption coefficients in square meters per kilogram and multiply it by specific masses of so many kilograms per square meter. Let me know if you want those figures.
[[Re: 329 “For water vapor, the absorption coefficient in the 12-25 micron range is 0.69 m-1 atm-1, so for a typical water vapor concentration of 0.4%, the transmission path length is 1.7 kilometers.”
Is this ln I/Io = – abc with I/Io = .01, a= 0.69 m-1 atm-1, b = 1.7 km, and c = 0.004 atm? ]]
Yes, essentially. I’m using T = exp(-k p L) where T is dimensionless transmissivity, k absorption coefficient in reciprocal meter atmospheres, p partial pressure and L path length. k p L is the (dimensionless) optical thickness, of course, so this is just a more elaborate way of expressing the old T = exp(-tau) equation, the classic definition of optical thickness.
Let’s review the original issue on this post:
“Without exception, the reconstructions show that Northern Hemisphere temperatures are now higher than at any time during the past 1,000 years (Figure 1), confirming and strengthening the conclusions drawn in the previous IPCC report of 2001.”
There really isn’t any valid scientific objection to this current estimate – there are no paleoclimate studies that contradict this statement. The current retreat of the cryosphere also provides observational evidence that supports the paleostudies.
As far as the water vapor feedback response, that’s been discussed many times on blogs, as have clouds. You can also read papers such as http://isites.harvard.edu/fs/docs/icb.topic53366.files/HeldSoden06JC.pdf
As far as Alastair’s comments: “All I can say is that no doubt you will find it difficult to accept that Robin McIlveen, Sir John Houghton, Keith Shine, Gavin Schmidt and Ray Pierrehumbert have all got it wrong. But as far as I can see that is the case.”
Right – but the case is that for Alastair to be right, every physicist who has studied radiative transfer in the atmosphere over the past fifty years must have got it wrong as well, and that also applies to Einstein and Subrahmanyan Chandrasekhar. It must be a massive conspiracy.
I have looked in the record of SH, NH and the Global record here, http://cdiac.ornl.gov/trends/temp/lugina/data.html. I have also looked at the UK and Swiss records.
I looked at the June to June record to attempt to see this large change in temperature in June91-June94. Zilch, nadan, nothing there at all. The record from June1991to1994, was a bit colder than the 25 year average, when the slope is taken into account, but so what so was 1984-1986, but big deal.
The big deal is this, I have heard quotes that the delta T was greater than half a degree, and so validated the forcing used in models. Now I don’t know how people managed to worse case of minus 0.22 degrees into more than minus 0.5 degrees, but I can guess. Here is my guess. Some one modelled what the temperature would be IF the mountain hadn’t done the big firework, THEN they took the actual temperature away from the one the calculated, and got a figure of Delta T of more than 0.5 degrees.
So would someone please give me the citation for the large delta T from 1991-1994 and tell me how it was calculated. It may be that I am a very cynical biochemist, BUT I do know how to read data.