Much research effort over the past years has gone into reconstructing the temperature history of the last millennium and beyond. The new IPCC report compiles a dozen reconstructions for the temperature of the Northern Hemisphere (including of course the original “hockey stick” reconstruction, despite opposite claims by the Wall Street Journal). Lack of data does not permit robust reconstructions for the Southern Hemisphere. Without exception, the reconstructions show that Northern Hemisphere temperatures are now higher than at any time during the past 1,000 years (Figure 1), confirming and strengthening the conclusions drawn in the previous IPCC report of 2001.
Fig. 1: Figure 6.10 (panel b) from the paleoclimate chapter of the current IPCC report (see there for details).
“Climate sceptics” do not like this and keep coming up with their own temperature histories. One of the weirdest has been circulated for years by German high-school teacher E.G. Beck (notorious for his equally weird CO2 curve). This history shows a medieval warm phase that is warmer than current climate by more than 1 ºC (see Figure 2). So how did Beck get this curve?

Fig. 2, modified from E.G. Beck (we added the green parts).
The curve is a fake in several respects. It originally is taken from the first IPCC report of 1990: a scan of the original is shown in Figure 3. At that time, no large-scale temperature reconstructions were available yet. To give an indication of past climate variability, the report showed Lamb’s Central England estimate. (Unfortunately this was not stated in the report – an oversight which shows that IPCC review procedures in the early days were not what they are now. We will post in more detail on the history of this curve another time.)
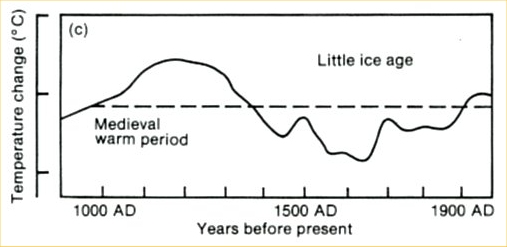
Fig. 3. The past millennium as shown in the first IPCC report of 1990, before quantitative large-scale reconstructions were available. This curve was based on Lamb’s estimated climate history for central England.
But Beck did not stop at simply using this outdated curve, he modified it as highlighted in green in Figure 2. First, he added a wrong temperature scale – the tick marks in the old IPCC report represent 1 ºC, so Beck’s claimed range of 5 ºC exaggerates the past temperature variations by more than a factor of three. Second, the original curve only goes up to the 1970’s. Since then, Northern Hemisphere temperatures have increased by about 0.6 ºC and those in central England even more – so whatever you take this curve for, if it were continued to present, the current temperature would be above the Medieval level, as in the proper reconstructions available today. As this would destroy his message, Beck applied another fakery: he extended the curve flat up to the year 2000, thereby denying the measured warming since the 1970s. With this trick, his curve looks as if it was warmer in Medieval times than now.
When approached directly about these issues, Beck published a modified curve on a website. He changed the temperature range from 5 ºC to 4.5 ºC – but he shortened the arrow as well, so this was just cosmetics. He also added instrumental temperatures for the 20th Century at the end – but with his wrong temperature scale, they are completely out of proportion. (In fact his version suggests temperatures have warmed by 2 ºC since 1900, more than twice of what is actually observed!)
Beck goes even further: in a recent article (in German), he has the audacity to claim that his manipulated curve is right and the more recent scientific results shown by IPCC are wrong. And for years, he has offered his curve on an internet site (biokurs.de) that distributes teaching materials for schools, with support from German school authorities. It is quite likely that his fake curve has been shown (and will continue to be shown) to many school children.

Error Correction from receently sent comment:
“It is strange what weather we have had all this winter; no cold at all, but the ways are dusty and the flies fly up and down, and the rosebushes are full of leaves; such a time of the year as never was known in this world before here.
Samuel Pepys’ diary, England, January 1661. A hundred years later in winter the Thames froze.
#192, DocMartyn, William gave an inline reply pointing out the misunderstanding underlying your first two questions there.
I urge you — as I did in reply to your #181 —-read the AIP History. Your questions are those asked in the 1930s and earlier, before most of what we know.
You also asked,what happened to the C14 from nuclear tests, then stated your belief that it’s all disappeared.
Why do you believe that? I find no support — except statements by creationists visiting physics forums — for that belief.
You can look this stuff up.
http://www.radiochem.org/paper/JN62/jn6206.pdf
Temporal Variation of Carbon-14 Concentration in Tree-ring Cellulose for the Recent 50 Years
Journal of Nuclear and Radiochemical Sciences, 2005
http://www.llnl.gov/tid/lof/documents/pdf/312713.pdf
Discussion: reporting and calibration of post-bomb 14 C data
PJ Reimer, TA Brown, RW Reimer – Radiocarbon, 2004
Look for articles with footnotes that give sources, not just statements of belief. What are your sources?
re 155: Tim Chase wrote: (We know that it is our carbon dioxide which has been entering the atmosphere because of isotopic analysis – the carbon we put into the atmosphere has an extra neutron – making it heavier…
Hi Tim, I’m not challenging you…just asking for clarification.
My understanding about how we know that the increasing CO2 (carbon dioxide) is anthropogenic (from human sources) from carbon isotope analysis (not to mention other ways as well) is the following from memory:
1) The heavier carbon 14’s nucleus is no longer radioactive in the atmospheric samples in question. (ie. the half-life has expired so that the carbon is well over ~70,000 years old…ie not going through the short carbon cycle of oceans to air to oceans or to biomass like plants trees soil, etc…ie. it comes from fossil fuel sources.
2) The carbon 12 to carbon 13 ratio is *lighter* toward more carbon 12 because the carbon’s source is plant based (fossil fuel based because plants have enzmyes that selectively exclude a lot of the heavier C13 and most fossil fuels come from plants) instead of more heavier carbon 13 which would be volcanic in origin (from Earth’s mantle).
So how do you get “heavier” CO isotopes showing the CO2 is anthropogenic…or did I miss something?
If I have missed something about the carbon 14 or something is off about my explanation above…please let’s discuss it.
Thanks,
So how do you get “heavier” CO isotopes showing the CO2 is anthropogenic…or did I miss something?
The evidence actually is that the 13C/12C ratio in the atmosphere has been declining, as I understand it.
It’s my understanding that deep ocean carbon is also depleted in 13C (due to the operation of the oceanic biological carbon pump), so the argument should really include a reason why increased upwelling/outgassing from the deep ocean can’t be the reason for increased atmospheric CO2 (not that I think it is, just that the possibility needs to be addressed in the argument).
Earlier I had mentioned various effects of glacier melt – the practical importance of glaciers – but I lacked figures. The following, which simply focuses upon the glaciers of Tibet should give us some sense of the magnitude of the problem.
With regard to flooding caused by glacier melt (in some places), I would suggest the following:
*
With regard to water shortages and their effects upon people, agriculture, and ecology, one might start with the following:
… and here is something more recent:
Global warming: Tibet’s lofty glaciers melt away
Research by scientists shows that the ice fields on the roof of the world are disappearing faster than anyone thought.
By Clifford Coonan
Published: 17 November 2006
The Independent/UK
http://environment.independent.co.uk/climate_change/article1990381.ece
It has been estimated that the glaciers of Tibet are being cut in half every decade. It also appears to be the case that they strongly influence global climate patterns. I myself will be digging into this some more in my own personal reading.
Paul Dietz #203 wrote:
Richard (#202), I don’t mind being challenged, and Paul, I don’t mind being corrected. I was simply under the impression that the reason why C13 was a marker for human emissions was that plants prefer C12, but if I am wrong, then it means that I have learned something.
>202, 155
Tim Chase had that backward when he wrote in #155 “the carbon we put into the atmosphere has an extra neutron …” if he meant fossil fuels. That would describe carbon-14, produced by hydrogen bomb tests in excess of the background produced by cosmic rays.
See this earlier thread:
https://www.realclimate.org/index.php?p=81
16 Dec 2004 How do we know that recent CO2 increases are due to human activities?
“… a very nice record of atmospheric 13C variations through time, and what we find is that at no time in the last 10,000 years are the 13C/12C ratios in the atmosphere as low as they are today. Furthermore, the 13C/12C ratios begin to decline dramatically just as the CO2 starts to increase — around 1850 AD. This is no surprise because fossil fuels have lower 13C/12C ratios than the atmosphere.
“The total change is about 0.15%, which sounds very small but is actually very large relative to natural variability. … .”
and many other examples
http://www.springerlink.com/content/mw3850g185v8tvp1/
Hank Roberts (#207) wrote:
That is what I was looking for. I stand corrected – and I am grateful.
Thank you, Hank.
Richard Ordway (#203) wrote:
This is probably what I was mis-remembering within the context of evolution – specifically with respect to carbon dating fossils, although it is actually the radioactive decay of C14 which comes into play. Now everything fits together. Fossil fuel from plants, plants preferentially uptake the lighter C12 – and bingo! The smoking gun. Or would that be a tailpipe?
Hi Tim,
If I remember correctly, at least one peer-reviewed study also mentioned that there is a high probability that the exact C13 to C12 ratio is a signature for each individual plant type (ie. ferns, palms, etc)!…so perhaps fossil fuel burning has a signature C14 to C12 ratio as well…anyone?
Oops,
I meant C13/C12 ratio above not C14 to C12. Sorry.
Re #203, 204, 207, 208, 209
Part of the reason why I picked Real Climate is because I tend to move quickly, perhaps a little too quickly, and not as systematically as I probably should. As such, I will tend to over-extend myself. At times I will make mistakes. In the past, I have belonged to forums where I needed to be corrected, but no one had either the expertise or willingness to do so.
I suspected that Real Climate would be different. I am glad to see that, at least with respect to this, I was right.
Dear Hank,
“#192, DocMartyn, William gave an inline reply pointing out the misunderstanding underlying your first two questions there.
I urge you — as I did in reply to your #181 —-read the AIP History. Your questions are those asked in the 1930s and earlier, before most of what we know.”
The very simple questions I asked have not been answered. I ask again;
What is the relationship between absorbance of a photon by CO2 and :-
A) A doubling of the CO2 concentration. So that at 380 [CO2] ppm; 50% of photons are absorbed, it follows that at 760 [CO2] ppm then X% of photons will be absorbed.
B) The attenuation of absorbance through the atmosphere at 380 [CO2] ppm, so that overall there is an absolute absorbance of 50% of the photos at a particular weavelength. What is the relationship between distance from the earths surface and the absorbance?
I am neither stupid, nor poorly read. I wish someone on this site to state an answer to these very simple questions.
“You also asked,what happened to the C14 from nuclear tests, then stated your belief that it’s all disappeared.
“Why do you believe that? I find no support — except statements by creationists visiting physics forums — for that belief.”
I was under the impression that the majority of the posters here would be rather well informed as to the isotope record and so neglected to give any links. However, I will show two data set representations, from Germany and New Zealand.
http://cdiac.ornl.gov/trends/co2/graphics/cent-scgr.gif
http://www.niwascience.co.nz/rc/prog/greenhouse/info/3
It can be seen that since the Test ban treaty, the levels of 14CO2 in the atmosphere are declining. It is also true that there will be some dilution effect from the addition of Human generated CO2 injected into the atmosphere over the same time period. However, ignoring the human part for a moment, it is obvious that the atmospheric half-life is about 12 years, consistant with my estimate for an average half-life of 9.2 years for CO2.
http://i179.photobucket.com/albums/w318/DocMartyn/Atmosvsinput.jpg
The dilution artifact may extend this by a maximium of about 6 months, by a back of the envelope calculation, but the mixing artifact of injecting 14CO2 into the upper atmosphere, during the H-Bomb tests themselves, is probably greater than this. When I have a little more time I will model it more acurately.
BTW “except statements by creationists visiting physics forums”, do you not think that statement was insulting?
And as to why fossil fuels have a low 13C/12C ratio — it’s because the carbon was selected by living organisms that favor C12 slightly over C13.
http://www.nsf.gov/news/frontiers_archive/3-97/3rocks.jsp
http://www.bio.net/hypermail/plant-biology/1994-February/002619.html
Astronomers pay close attention to that C12/C13 ratio in stars and comets; both are produced in fusion reactions.
The Isotope Ratio C^{12}/C^{13} in a Comet
A Stawikowski, JL Greenstein – The Astrophysical Journal, 1964
http://www.journals.uchicago.edu/cgi-bin/resolve?id=doi:10.1086/148023
Both isotopes are stable (they don’t fission spontaneously); changes in the ratio occur in living systems:
Plants do selectively favor C12 over C13; metabolic paths also differ; the C12/C13 ratio distinguishes cane sugar from beet sugar (both sucrose))
http://links.jstor.org/sici?sici=0006-3568%28197204%2922%3A4%3C226%3ANAOTSI%3E2.0.CO%3B2-G&size=LARGE&origin=JSTOR-enlargePage#abstract
The ratio is also used to distinguish endogenous mammalian testosterone from supplements made from plant sources (in drug testing for athletes)
R Kazlauskas, G Trout – Therapeutic Drug Monitoring, 2000 – drug-monitoring.com
… The most commonly used endogenous steroid has been testosterone (T) … T used in pharmaceutical preparations has a lower C12/C13 ratio than naturally …
[I’d double check wherever you find the ratio stated: C13/C12 is not the same as C12/C13, but don’t assume secondary sources got it right — hr]
Those living organisms have pre-selected the carbon that ends up buried and changed over time into fossil coal and petroleum.
Watch carefully where religious beliefs get involved; the physics of radiocarbon dating
http://www.c14dating.com/int.html
causes problems for some people’s belief systems, and you’ll find a whole lot of beliefs stated as facts in discussions about carbon isotope issues in physics.
For example, freshwater mussels are very low in carbon-14 –you can look this stuff up: http://www.ncseweb.org/resources/articles/8052_issue_08_volume_3_number_2__12_4_2002.asp#Answers%20to%20Creationist%20Attacks%20on%20Carbon-14%20Dating
Always ask and check the cites.
Trolls don’t footnote, perhaps the devil is in the details.
Paul Dietz, Re #204. I can think of 2 reasons and one test that show the carbon in the atmosphere is not coming from the Oceans. 1)Since the overturn time of water in the Oceans is of order hundreds to thousands of years, that would imply that at some point in the past few thousand years, the oceans got a big infusion of CO2. I don’t know where this would have come from except from the atmosphere, and we know that carbon in the atmosphere is higher than it has been in at least 650000 years. Second, if the carbon is coming from the oceans, the carbon content of the oceans ought to be decreasing–it is doing the opposite as indicated by the increasing acidity. The test would be to look at the C-14 to C-12 ratio. The water from the oceans ought to have at least some C-14, while that from fossil fuels ought to be 100% depleted. So it all depends on whether the C-14 in the atmosphere is decreasing as rapidly as expected given the C13 to C12.
Ray, Re #215: it’s not clear it would imply the oceans got a big CO2 infusion. Wouldn’t a sudden change in the rate of upwelling cause a sudden change in the rate at which CO2-rich water was brought up, for its CO2 to escape? This would cause a change in total oceanic CO2, but not necessarily in surface waters — it would cause a decline in CO2 stored in the deep ocean, somewhere, which one might argue has been missed.
14C would be the way to rule out this hypothetical release, since even deep ocean carbon will not have had all the 14C decay away.
Re 200 & 201 Bob Gardiner: “Climate sceptics invariably do not question the rise in temperature just the cause being put down to the wrong light bulbs being used, etc.”
They also invariably ignore the well understood science of the greenhouse effect, dismiss the nearly 38% increase in atmospheric CO2 from burning fossil carbon fuels, and fixate on past natural climate variability as an explanation for the current unnatural warming trend.
RE Decline of C14 in atmospheric carbon dioxide
I am at work at the moment, but here is a little material which might be helpful…
The Discovery of Global Warming
Roger Revelle’s Discovery
Spencer Weart
http://www.aip.org/history/climate/Revelle.htm
CARBON-14 MEASUREMENTS IN ATMOSPHERIC CO2 FROM NORTHERN AND SOUTHERN HEMISPHERE SITES, 1962-1993
NDP-057 (1996)
http://cdiac.ornl.gov/epubs/ndp/ndp057/ndp057.htm
DocMartyn, let me try to explain the decay of carbon-14 in the atmosphere. First, you are looking at the carbon content of the entire atmosphere–not just the CO2. So, we do not know what form the carbon was in. Some of it could have been soot or methane. However, even if it was CO2, how quickly an individual atom of carbon resides in the atmosphere is not that relevant. CO2 is chemically very stable. Even when it reacts with water to form H2CO3, the H2CO3 decays into H20 and CO2 again. OK, so if a CO2 molecule containing a C-14 atom goes into the water and becomes a carbonic acid molecule, another carbonic acid molecule will react to give another CO2 molecule. But there is much less C-14 in the oceans, so the new CO2 molecule will most likely be a C-12 or maybe a C-13. And the concentrations of CO2 and H2CO3 stay the same, because the surface of the ocean is in equilibrium with the atmosphere. The only way to get rid of a carbon atom for any length of time is for it to travel to the deep ocean–and that doesn’t happen that often. So, while the individual carbon atom will spend time in the atmosphere and in near-surface waters, the CO2 concentration will stay high. Even biomass yields its carbon back on a relatively short timescale–at most a few hundred years for old-growth forest.
Now, as to your questions.
A. I believe the probability of an IR photon in the carbon band escaping decreses roughly logarithmically with increasing CO2 content–thus double the content an the photon is about 70% less likely to escape.
B. OK, in effect you are asking the mean-free path. This reference is relevant:
http://atol.ucsd.edu/sio209_rad/stephens-rtebook/AT622_section4.pdf
The paper to get which first fully modeled and accounted for all the C14 released during the nuclear tests would be:
Duffy, P.B., and K. Caldeira, A three-dimensional model calculation of ocean uptake of bomb 14C and implications for the global budget of bomb 14C, Global Biogeochemical Cycles 9, 373-375, 1995.
Unfortunately I do not have it yet.
However, the basic problem with DocMartyn’s approach is that while he is correct that the “half-life” for carbon dioxide entering the ocean is roughly ten years, as was concluded before, he is not taking into account the carbon dioxide which is being released from the ocean – and as the carbon dioxide entering the ocean since the test ban is undoubtedly much smaller than the sink-source that it is entering, we shouldn’t expect to see the C14 consitute a particularly large fraction of the carbon dioxide leaving the ocean. Nevertheless, it is significant enough that it can be measured, and this has been invaluable in understanding the carbon cycle. For example, we know that some boreal forests are now net emitters of carbon dioxide. Moreover, we will now be in a position to determine when the ocean becomes a net emitter.
With regard to the boreal forests, please see:
Two more links which may be of interest:
The Discovery of Global Warming
The Carbon Dioxide Greenhouse Effect
http://www.aip.org/history/climate/co2.htm
Letters
More Notes on Global Warming
May 2005, page 16
http://www.physicstoday.org/vol-58/iss-5/p16a.html
DocMartyn–
Look at this search: http://www.google.com/search?q=c14+decline+atmosphere
This is what people are reading online.
First hit, I recommend and will quote from below.
Second and third are from creation-science-prophecy.com. That’s Google search rank, it’s what people are reading.
You’re confusing the rate of cycling of CO2— balanced in the atmosphere and oceans for millenia — with the removal of a huge sudden increase in C14, which is diluting into oceans that had only a tiny natural background amount.
Consider the proportions. .
C14 increased perhaps 5x from surface fusion bomb testing in just a few years. Of course C14 disappears rapidly.
C12/13 have been in balance for millenia, and we’ve added a few percent more in excess in recent centuries — about half the excess from fossil fuel is accumulating in the atmosphere and the oceans, because natural sinks aren’t removing it.
See the difference?
This may help:
http://yarchive.net/chem/carbon_14.html
“From: rparson@spot.Colorado.EDU (Robert Parson)
Newsgroups: sci.chem
Subject: Re: Global Warming Higher Than Expected
Date: 16 Jul 1999 16:57:49 GMT
In article <7mdmqv$odk$1@node2.nodak.edu>, wrote:
Superdave the Wonderchemist
>
>Interesting… So what is the 14-C dilution factor? Fossil fuels contain
>almost no 14-C (since they’ve been underground for too many millions of
>years). Therefore, C-14 dilution should be a wonderful way to determine
>anthropomorphicly induced atmospheric CO2 increases.
Indeed it is – it even has a name, the “Suess Effect”, after the
geochemist who first detected it in the 1950’s. The original reference
is H. Suess, _Science_ _122_, 415 (1955); a more recent one is
M. Stuiver and P. D. Quay, “Atmospheric C-14 changes resulting from
fossil fuel CO2 release and cosmic ray flux variability”, _Earth and
Planetary Science Letters_, _53_, 349, 1981. Figure 2 of this paper has
exactly what you’re looking for, tree-ring derived atmospheric C-14
from 1820 to 1954. There is a clear secular decline in Delta-C-14
of about 25 per mil betweenm 1900 and 1950.
As others have remarked, the nuclear tests of the 1950’s, which doubled
atmospheric C-14 over a period of a decade, throw a monkey wrench into
this analysis. Since the cessation of the tests in ~1963 atmospheric
C-14 has been rapidly declining, with Delta-C-14 dropping from nearly
twice the pre-bomb level in 1965 to about 20 percent above the pre-
bomb level in 1990. (R. Nydal and J. S. Gislefoss, _Radiocarbon_ _38_,
389, 1996). This decline is due primarily to atmosphere-ocean exchange
the effect of fossil-fuel dilution is in the noise by comparison. (In
fact, the atmospheric C-14 decline is one of the primary methods for
measuring the rate of carbon exchange between air and seawater, a very
complicated problem involving nasty multiple ionic equilibria plus
the effects of wind speed and ocean temperature. If you want somebody
who _really_ understands ionic equilibria in aqueous solution, look for
a chemical oceanographer.)
RE #128 and my previous post #51
I too noticed that the general trend in IPCC report matched Beck’s and showed a ~1 degree variation even after elminating the last hundred years. My question was what accounted for this?
According to IPCC, it is volcanism and solar variation.
However, there is a question of how much variation really occurred. When we look at the graph, it is only the purple lines that show the ~1 degree variation. The other studies show much less variation.
Either I missed it or didn’t understand, but I can’t tell from IPCC report what accounts for the variation in the studies. I can see that they used different proxies but are some better than others? Are we to average out the studies? Is this just a judgment call and nobody really knows?
Also, what would happen if we extended the global temperature graph for the entire Holocene? Is this possible? I thought I read that 8,000 years ago was warmer than today?
Absorption of light along a path is governed by Beer’s law
ln (I/Io) = – sigma N L where Io is the incident intensity, I the intensity at the end, sigma the absorption cross-section (a function of wavelength), N the number density and L the path length
(see any elementry physics or chemistry text) however that does not account for emission, for that see Kirchhoff’s law.
“The very simple questions I asked have not been answered. I ask again;
What is the relationship between absorbance of a photon by CO2 and :-
A) A doubling of the CO2 concentration. So that at 380 [CO2] ppm; 50% of photons are absorbed, it follows that at 760 [CO2] ppm then X% of photons will be absorbed.”
Meaningless as stated because you do not specify the length of the path or the wavelength. At atmospheric pressure and 380 ppm on a strong line this is a few meters.
“B) The attenuation of absorbance through the atmosphere at 380 [CO2] ppm, so that overall there is an absolute absorbance of 50% of the photos at a particular weavelength. What is the relationship between distance from the earths surface and the absorbance?”
The absorption is much stronger that this, but there is also emission on the same lines from CO2 in the atmosphere.
“I am neither stupid, nor poorly read. I wish someone on this site to state an answer to these very simple questions.”
You need to learn about spectroscopy and radiative processes. You also need to tone it down. It is hopeless to try bullying people who know about things you are ignorant about.
#216, Paul, a change in ocean upwelling of that magnitude would have other observable effects–most notably a drop in temperature of the surface waters–again, the opposite of what we are seeing. Keep in mind that the change would have to be sustained and to follow an exponential trend–both quite unlikely.
Note that C14 measures vary from site to site; here’s a summary for the Pacific Ocean:
http://geoweb.princeton.edu/people/resstaff/key/woce/C14.Changes/key.html
Also very relevant and interesting: http://www.crafoordprize.se/download/18.51ddd3b10fa0c64b24800021404/Craf_adv06.pdf.
18 January 2007
Advanced information on the Crafoord Prize in Geosciences 2006
Earth�s operation as a chemical, physical, and biological system
Recommended reading. The article concludes:
“… Broecker has been a strong and active teacher of his subject and an
educator, producing accessible and engaging books and articles that can capture the
attention of the general public and students, while avoiding the trap of �dumbing
down� the subject to make it palatable to non-specialists. Annoyed by the high prices
charged by publishers of both journals and books he has, since 1982, been publishing
his own works at cost price, via the “eldigio” press, to make them available to the
widest possible readership. Several of his books and articles have served to present
the basic science with great clarity to a non-specialized audience (Broecker 1974;
Broecker 1985; Broecker 1992a, b), while another (Broecker and Peng 1982) has been
the classic chemical oceanography textbook for almost 25 years.”
Perhaps worth adding to “Start Here” as well?
Being in that class, I found Timothy’s words on skeptics (199) to be quite good. I would like to tweak it a bit, though. The “disbelief” of the listed points might not be so absolute. E.g., I don’t disbelieve the radiative absorption/re-emission properties of GHGs, like CO2; but I question (am skeptical of) the precise mathematics and observations that make up the forcing and temperature quantifications from it. There is likely more granulation in the degree of skepticism. I guess I would call myself “not convinced” as opposed to “skeptical”, but that’s getting too refined for practicality — just plain skeptical is easier.
The referenced Pyrrho story is meaningful, but cuts both ways. It made no mention of the cost of Pyrrho pulling his instructors head out of the mud (though implied it was virtually zero cost.) One post a while back was chastising skeptics for just looking out for our economics interests. Well, DUH! Now I’m not a skeptic because of that, but it is a major reason why I press the skepticism hard. Despite the sanguine economic prognostications offered (often by physicists… [;-) by many, it’s not clear in the least that reducing our carbon emissions by magnitudes in a couple of decades won’t wrack economic havoc. To the extent possible the science and arguments of AGW needs to be exercised and tested excruciatingly. (A democratic vast majority doesn’t come even close!) But I also have to admit the other side, which causes me angst from time to time. Like while Pyrrho thought about it, his teacher (presumably) died, there is the prospect that the AGW boys (and girls) might prove to be right, but after finally satisfying us well-intentioned skeptics it’s now too late to recover and we all die (or somethin’). Just can’t win for losing.
Rod B. One way I would distinguish between a skeptic and a denialist clothed in the robes of a skeptic is that a skeptic could be convinced if they saw some critical missing piece of information. A denialist would rationalize any new information and remain “skeptical”. They often would not even make an effort to understand the new information. So I ask, Rod, what would it take to convince you?
Rod, are you familiar with the term ‘sunk costs’? It’s part of the evidence against the faith that markets are rational.
It refers to the problematic behavior of sticking with a bad investment, ‘riding it down’ — once people have ‘invested’ (sunk) so much wealth (cost) in their choice, they have trouble letting go of it even when it’s known bad.
It’s typical human behavior — it takes a rare and disciplined understanding of economics to avoid it.
People generally have enormous trouble recognizing that something they invested a lot of money in can be worth a lot less than they thought it would be — the costs are sunk, lost, gone.
The ‘monkey puzzle trap’ is supposedly a large nut in a small-mouthed heavy jar, so the monkey can see it, reaches in, grabs it, then can’t get its closed fist out of the narrow opening and is trapped by holding on, unable to let go even as the hunter approaches. That may be apocryphal — we know people have this problem, but I don’t know that any other animal does.
The costs we didn’t know about — externalized, not accounted for — from fossil fuel use are like that. But the time span is longer than a human lifetime so the pain comes mostly for the next generation after we’ve taken the pleasure.
It’s real —the losses are _known_ continuing for several hundred years more just from the committed change already in the climate system. One costs are sunk and recognized as lost, there’s nothing smart to do but believe the loss and walk away. That’s our problem here.
It appears that we have some agreement, the rate of atmospheric [CO2] efflux (to all sinks) is approximately 0.076 year-1; so that 50% of the CO2 enters the Earths sinks at 9 years and 90% is gone at 30 years. Of course the system is a pseudo-steady state and so influx into the system occurs at the same rate.
We also know that the steady state atmospheric CO2 concentration was about 280 ppm before humans began to inject large quantities of CO2 into the atmosphere, so that the natural background influx (from all) was about 21 GT year-1, current emissions are a third of this value.
Now all we need to know is if these sinks can be saturated. There are two ways to do this; a plot of anthropogenic CO2 vs. atmospheric CO2 would be non-linear if we were saturating the various CO2 sinks; however the line is linear indicating that the Earths various sinks are not saturated.
The second way is from the disappearance of C14 from the atmosphere. The disappearance of 14C from H-bomb testing shows that the flux into the atmosphere of 14CO2, captured from the Earth carbon sinks, is as small as to be trivial; therefore the CO2 buffing capacity of the Earths CO2 sinks is more than two orders of magnitude greater than the rate at which CO2 is removed from the atmosphere. The Earths sinks are nowhere near saturation, indeed the system can be described as a simple steady state system where the natural rate of atmospheric CO2 influx into the atmosphere is 2 GT per year and the efflux into the earths sinks is 0.076 year-1, supporting a natural atmospheric [CO2] of 280 ppm. Such a model also indicates that to reach 2xCO2 (560 ppm) will occur when human CO2 emissions reaches approximately 20 GT per year.
————————————————————————
It also appears that the majority of members of this debate now accept that CO2 atmospheric absorbance follows the Beer-Lambert Law, which is encouraging.
———————————————————————–
“You need to learn about spectroscopy and radiative processes. You also need to tone it down. It is hopeless to try bullying people who know about things you are ignorant about.
Comment by Eli Rabett â�� ”
I have been doing spectroscopy for more than 20 years. I do not bully, I just hate when people do not answer simple questions. I also hate complicated maths, when simple maths will do.
[[“It is strange what weather we have had all this winter; no cold at all, but the ways are dusty and the flies fly up and down, and the rosebushes are full of leaves; such a time of the year as never was known in this world before here.
Samuel Pepys’ diary, England, January 1661. A hundred years later in winter the Thames froze. ]]
Yes, and that shows why it’s not good to generalize from too small a sample. One warm winter doesn’t prove much. On the other hand, for the present global warming, we have 120+ years of time series data and proxies going back, in some cases, 650,000 years. That’s a fair sample.
[[What is the relationship between absorbance of a photon by CO2 and :-
A) A doubling of the CO2 concentration. So that at 380 [CO2] ppm; 50% of photons are absorbed, it follows that at 760 [CO2] ppm then X% of photons will be absorbed.]]
One CO2 molecule is only likely to absorb one photon at a time. When there are more CO2 molecules in the air, more photons are likely to get absorbed.
Most of the radiation from the ground is absorbed fairly close to the ground (i.e., in the first few kilometers). But absorption of the ground IR isn’t the only factor involved. Absorption between layers of air is important as well, and it’s easy to show that even if all the IR from the ground was absorbed by the lowest level of air, more CO2 in the air would still heat the ground further. Let me know if you want the explanation as to why.
Alright, time for me to show my ignorance.
All this talk about CO2 and photons and other such things — isn’t it more likely that these photons, after being absorbed and emitted and reabsorbed and reemitted will simply escape into space, even if only because of the curvature of the atmosphere and the simple fact that there’s less air up there to absorb all those photons? Dittos for the longwave radiation given off by condensation of water vapor, even moreso because the moisture content of air increases exponentially with temperature.
This probably should have been asked when we were talking about simple models, but this is the hot thread today :)
DocMartyn, I’m sorry, but that is just flat wrong. How on Earth do you get that atmospheric CO2 is rising linearly? Look at this graph:
http://www.globalwarmingart.com/images/5/52/Carbon_History_and_Flux_Rev.png
Now go ahead and just try to fit a straight line to that–that’s an exponential rise. Here’s a caveat: Never judge the form of a curve based on too short a data set. Remember the taylor series for the exponential: e^x=1+x+x^2/2!+… For very small x, the linear terms dominate–but that doesn’t mean the trend is linear.
Second, your reasoning on the C-14 is just flat wrong. First, the influx of C-14 from the bomb tests can be viewed as an impulse source, that rapidly doubles the C-14 in the atmosphere. Now what will happen? Let’s look at one sink to simplify–the oceans. There’s 2x the C-14 in the atmosphere as is the equilibrium level with the oceans, so the net flux of C-14 will be toward the oceans–UNTIL YOU NEAR EQUILIBRIUM. Then the flux will stabilize. That does not mean that you aren’t still getting an atom of carbon into the atmosphere (or rather, 90 atoms of carbon for 92 into the oceans). It just means that only about one in a trillion will be C-14. So:
1)CO2 in the atmosphere is increasing exponentially; and
2)The sinks are saturating, and will be moreso as temperatures rise.
I do not bully, I just hate
You can get help for that. There are public services available.
FurryCatHerder,
Reradiation is not the only possible outcome, the IR radiation excites a vibrational state of CO2, and this can couple electromagnetically or mechanically to the mechanical motion of adjacent atoms. Also, keep in mind that the re-emission can be in any direction over 4-pi steradians–so on average half the radiation gets sent back toward the ground, where it can again be absorbed. (Remember the atmosphere is only about 100 km thick, so even at the top of the angle, Earth subtends nearly a 2-pi Sr angle.) In essence, the atmospheric CO2 becomes a new source of radiation incident on Earth.
Re DocMartyn #230 (sequestration of carbon)
Given your approach, I would like to know how exactly you interpret the chart on pg 3 of the following:
Tracing the Role of Carbon Dioxide in Global Warming
Science & Technology Review March 1998
http://www.llnl.gov/str/pdfs/03_98.2.pdf
What I see is the level of C14 being relatively constant prior to the bomb tests, a large pulse entering the system, and the C14 approaching a new level which approximates an asymptotic path. Just eye-balling it, I suspect that just about anyone will see something odd: the level which it appears to be approaching asymptotically is substantially higher than the relatively constant level it was at prior to the bomb tests.
Dear Timothy Chase, that is exactly what one would expect. The rate at which 14C is produced is typically dependent on the amount of N2 in the atmosphere and the amount of cosmic ray radiation. Though the latter varies, it means that the level of 14C in the Earths various CO2 sinks would have been in a steady state before mankind started burning fossil fuel and exploding thermonuclear devices in the atmosphere.
H-Bomb testing produced an atmospheric spike of 14CO2, puterbing the steady state distribution beween the various sinks. This purtubation quickly (3 decades) reverted to steady state again. Exactly what one would predict of there was rapid exchange between the various sinks.
Dear Ray Ladbury
“Also, keep in mind that the re-emission can be in any direction over 4-pi steradians–so on average half the radiation gets sent back toward the ground, where it can again be absorbed. (Remember the atmosphere is only about 100 km thick, so even at the top of the angle, Earth subtends nearly a 2-pi Sr angle.) In essence, the atmospheric CO2 becomes a new source of radiation incident on Earth.”
True, however the concentration of CO2 in the atmosphere is inhomogenous, there is much more at the surface and little at the top, where the photons are ultimately lost to space. What we have is a case of non-equlibrium theromdynamics. Space is a sink for photons and the surface is not. Photons that migrate downward encounter more CO2 molecules and those that go upward less. If you do a random walk deffusion type analysis you will note that the overall transfer is from the surface and into space; the sink will always win.
The question is what effect will the CO2 have on heat trapping? Will an increase in CO2 from 280 ppm to 560 ppm have a large effect on the surface temperature. The answer has got to be no, given that the effect will be about a change in the trapping efficency of about 12%.
Gavin, in the link in 237, there’s a model prediction re C14; can you comment on that one? It’s testable, interesting.
Hank Roberts (#239) wrote:
You mean the part where the carbon dioxide which we are putting into the atmosphere begins to flush the radiocarbon out of the ocean so that there is a net influx of the radiocarbon into the atmosphere from the detonations prior to the test ban? I was wondering when someone would notice.
DocMartyn (#238) wrote:
Doc, you appear to be seeing only that half of the chart (see #237) which you want to see. What I was specifically pointing to was that the equilibrium level of radiocarbon from before the tests will not be reached again except in geologic time. There is only so much of the pulse that the ocean will absorb.
And as a matter of fact, there will soon come a point at which there will be a net efflux of radiocarbon from the ocean into the atmosphere – our emissions will be flushing it out. Then at some point, perhaps only a handful of decades from now, there will be a net efflux of carbon dioxide from the ocean as the rise in global temperature causes the ocean to become an emitter.
Sure – early on the actual curve approximates the hypothetical exponential decay of the pulse from the atmosphere which you envisioned. But it is also true that over a suitably short extension of a curve, line will approximate that. But for this to follow the kind of exponential decay (even in approximation) that you envisioned, the latter part of the curve would have to be settling down close to the original level.
It isn’t.
re Ray Comment 234
“DocMartyn, I’m sorry, but that is just flat wrong. How on Earth do you get that atmospheric CO2 is rising linearly? Look at this graph:
http://www.globalwarmingart.com/images/5/52/Carbon_History_and_Flux_Rev.png
Now go ahead and just try to fit a straight line to that–that’s an exponential rise”
If you look at the graph I presented would would have noticed that I plotted Human release of CO2 in GT on the x-axis and atmospheric [CO2] ppm, at yearly peak and trough, on the y-axis.
Now the intercept of this plot should give the historic atmospheric [CO2], even though the data set doesn’t include it. The plot at x=0 is about 280 ppm, so, so-far so-good.
The gradient of the slope gives 1/CO2 atmospheric efflux rate year-1. Which is 0.076 or a half-life of 9.2 years. This value is very close to the rate at which H-bomb generated 14CO2 disappeared from the atmosphere following the Test ban treaty, so again, so-far so-good.
From the intercept at x=0 and the efflux rate we can calculate that the “natural” CO2 influx rate into the atmosphere, independent of human activity, is about 21 GT per year.
———————————————————————–
The difference between atmospheric [CO2] ppm in the spring and autumn is also quite interesting. Does anyone have the average sea surface temperatures, per area, for the whole planet in April and October?
From this data I could estimate the effect of global sea temperature changes on the level of atmospheric [CO2].
RE: 230 It is implied that one obtains 21 GT CO2 year-1 for the natural background influx based upon a steady state CO2 conc of 280 ppm and an efflux rate to all sinks of 0.076 year-1. I don’t understand how one can get any influx rate unless you have the total mass of the atmosphere.
DocMartyn #242 wrote:
I am sure you could – by neglecting anything beyond the local effects of the change in temperature. You need to look at the long-term behavior – not just the local tangent – or even an exponential curve which fits the first couple terms of local behavior. By looking at the seasonals, you are factoring out all of the long-term effects.
It takes a little bit of time for the relevant layer of the ocean to warm up. (This would be roughly the first meter – if one is thinking in terms of a few decades.) It has thermal inertia. Largely a function of its mass, I believe. Long-term effects require the ocean to warm up, not just the atmosphere.
Correction to my most recent post in response to DocMartyn’s #242 –
What I was recalling was the diffusion of CO2 into the ocean. But what is most significant to DocMartyn’s “seasonal analysis” of the effects of temperature upon atmospheric CO2 will be the rate of heat diffusion through the layers of the ocean. Global warming is detectable as far down as 1500 meters. But either is sufficient to invalidate his “seasonal analysis” – demonstrating that such a short-term analysis neglects the long-term effects.
DocMartyn,
You can use NOAA’s atlases (2005- readily available on line, just type in NOAA Atlas 2005 into Google) for all the sea surface temperature measuremnts you need, annual averages, seasonal averages, etc. Also they provide the same info for various water depths from the surface to 5500 meters. This is free access to all.
> the concentration of CO2 in the atmosphere is inhomogenous,
> there is much more at the surface and little at the top …
What do you mean by “concentration” there?
You’re not referring to Dr. Wegman’s answer to Rep. Schakowski, are you?
“Prof. WEGMAN: Carbon dioxide is heavier than air…. if the carbon dioxide is close to the surface of the earth, it’s not reflecting a lot of infrared back.”
RE: 243 Comment by Don Fontaine “I don’t understand how one can get any influx rate unless you have the total mass of the atmosphere.”
We know how much CO2 we added every year. We know the relation ship between the amount we add and the amount of [CO]2 in the atmosphere. If we extend the line so that y=0, the intercept point is equal to -Natural CO2 influx. Remember at steady state, influx equal efflux. So we can calculate the eflux at any point along the curve.
DocMartyn #242 wrote:
A few more questions regarding your proposed calculations…
Just out of curiousity, have you considered the seasonal nature of our CO2 emissions? Have you considered how the wind patterns change throughout different times of the year? Have you considered how unevenly distributed the CO2 emissions are? Would you be measuring the CO2 levels at Mona Loa? It takes time for such emissions to reach Mona Loa. Years, roughly on the order of a decade from some parts of the world, and it is through a process of diffusion. This would blur the seasonal effects of temperature upon CO2 levels if this were where you were performing your measurements.
And if not there, then where? And what about these other factors?
> Does anyone have the average sea surface temperatures, per area,
> for the whole planet in April and October? From this data I could
> estimate the effect of global sea temperature changes on the level
> of atmospheric [CO2].
There’s a gap in your logic there.
Look up the actual measurements of CO2 in the ocean. This is a big research area, lots published, and a real area of concern as ocean pH is changing fast.
http://www.princeton.edu/~mhiscock/OCCC.pdf
Eos, Vol. 86, No. 42, 18 October 2005
— excerpt—-
Ocean biogeochemistry is a critical component
of the Earth�s climate system, regulating
on timescales of decades to millennia the
atmospheric levels of carbon dioxide (CO2),
and other radiatively active gases. Since the
pre-industrial era, the ocean has taken up
about half of the carbon released by fossil
fuel combustion, partially mitigating climate
change.The future behavior of this oceanic
sink, however, is not well understood and remains
one of the major climate uncertainties
[Sarmiento and Gruber, 2002].
The ocean carbon inventory depends, in
part, upon the complex responses of the
natural ocean ecosystems and carbon system
to changes in ocean circulation, dust
deposition, ocean pH, ultraviolet radiation,
and other factors [Fasham, 2003].Addressing
this problem requires an integrated research
effort on a variety of fronts, ranging from
monitoring the temporal evolution of the
ocean inorganic carbon inventory to innovative
studies of poorly known biological and
chemical dynamics.
As part of the new Ocean Carbon and Climate
Change (OCCC) program [Doney et al.,
2004], (sponsored by the multi-agency U.S.
Global Change Research Program�s Carbon
Cycle Science Program), a science workshop
called The Ocean Carbon System: Recent
Advances and Future Opportunities was held
recently at the Woods Hole Oceanographic
Institution ….
More than 100 scientists participated ….
Electronic versions of many of the plenary
talks are available
[the published link is outdated; this will get you to the material –hr]
http://www.whoi.edu/search.do?q=OCCC+workshop&btnG=Search&ie=&site=WHOI_External&output=xml_no_dtd&client=WHOI_External&lr=&proxystylesheet=WHOI_External&oe=
… findings on biogeochemical cycling across
the air-sea interface, … the quantification of the oceanic
uptake of anthropogenic CO2 and the resulting
geochemical and ecological impacts due to
ocean acidification; and the application of new
technologies and methods to ocean biogeochemistry.
— end excerpt—–
Or you can look at the Mauna Loa information:
At Mauna Loa, in the annual variation, it’s explained — you’re looking at CO2 from photosynthesis in North America, primarily, lagged by the time it takes to arrive. This isn’t new.
See here: http://www.atmos.ucla.edu/~ben/PUBLICATIONS/Buermannetal2006.pdf
The changing carbon cycle at Mauna Loa Observatory
— excerpt—-
The amplitude of the CO2 seasonal cycle at the Mauna Loa Observatory
(MLO) increased from the early 1970s to the early 1990s but
decreased thereafter despite continued warming over northern
continents. Because of its location relative to the large-scale
atmospheric circulation, the MLO receives mainly Eurasian air
masses in the northern hemisphere (NH) winter but relatively more
North American air masses in NH summer. ….
…
The seasonal cycle of
atmospheric CO2 at the MLO, with a maximum at the beginning
of the growing season (May) and a minimum at the end of the
growing season (September/October), records the ��breathing��
of the northern hemisphere (NH) biosphere, that is, the seasonal
asynchrony between photosynthetic drawdown and respiratory
release of CO2 by terrestrial ecosystems (e.g., refs. 1â��3)….
…
Our analysis of the increasing trend in the
MLO amplitude from the early 1970s to the early 1990s extends
the analysis of Keeling et al. (1) by attributing the photosynthetic
drawdown to North America and enhanced cold-season respiration
to Eurasia. The time series of the MLO amplitude beyond
the early 1990s shows behavior and controls very different
from the earlier two decades. Our analysis suggests that throughout
the last two decades, the MLO CO2 seasonal amplitude has
recorded a changing North American carbon sink that is dominated
by shifts in the North American hydrologic regime rather
than by temperature trends. The decline in the MLO amplitude
since the early 1990s captures the effects of North American
droughts, especially those of 1998�2003, on growing-season
carbon uptake on the continent. The amplitude decline is also
attributed to reduced cold-season CO2 transport from Eurasia in
the early 1990s arising from changes in large-scale atmospheric
circulation, especially in the NH spring.
—- end excerpt—–
Direct measurement of CO2 in the ocean water is done routinely.
This is simple physical chemistry, not complicated mathematical modeling of radiation physics. Ocean pH is decreasing — that’s a problem independent of global warming and more urgent.
This may help:
http://www.terrapub.co.jp/e-library/kawahata/pdf/045.pdf
— excerpt ——
“A primary motivation for studying deep circulation is that the deep ocean is a major component of the global carbon cycle. The world ocean has approximately 38,000 Pg-C (1015 grams C), which dwarfs the atmospheric and terrestrial biospheric reservoirs that respectively have about 730 and 2200 Pg-C (Houghton et al., 2001). Since most of the oceanic carbon resides in the deep ocean, even a small change in its carbon budget can significantly impact the atmospheric budget and hence the global climate. Under natural conditions, the chances of such an event may seem remote, because the deep ocean is a slow component compared to the atmosphere, upper ocean, and terrestrial biosphere.
However, measurements from polar ice cores provide abundant evidence for
multiple, abrupt climate changes in the last glacial cycle (Dansgaard et al., 1993), some of which may have involved changes in the deep ocean (Broecker, 1998, 2003). Abrupt climate change may be a real possibility today, when human activities that modify the physical environment are increasing globally (Alley et al., 2003; Broecker, 1997).
Another important reason for accurately characterizing the deep ocean is the need to validate ocean carbon cycle models. These models are used frequently to predict the response of the ocean to increasing atmospheric CO2. Projections of future carbon uptake by the ocean (Houghton et al., 2001) inevitably involve the deep ocean. We would be hard pressed to place confidence in projections from any model that does not reproduce the modern deep ocean behavior reasonably well. In this work, we use the new measurements from the World Ocean Circulation Experiment (WOCE) conducted during the decade of 1990s to present for the first time objectively gridded global maps of deep natural 14C.
… The long time scale associated with the deep ocean circulation are readily appreciated when 14C abundance is converted to 14C age (Fig. 2). The difference of about 1000 years between the Atlantic and Pacific is the basis for characterizing the global overturning circulation as having millennial time scale.
—- end excerpt—-