Much research effort over the past years has gone into reconstructing the temperature history of the last millennium and beyond. The new IPCC report compiles a dozen reconstructions for the temperature of the Northern Hemisphere (including of course the original “hockey stick” reconstruction, despite opposite claims by the Wall Street Journal). Lack of data does not permit robust reconstructions for the Southern Hemisphere. Without exception, the reconstructions show that Northern Hemisphere temperatures are now higher than at any time during the past 1,000 years (Figure 1), confirming and strengthening the conclusions drawn in the previous IPCC report of 2001.
Fig. 1: Figure 6.10 (panel b) from the paleoclimate chapter of the current IPCC report (see there for details).
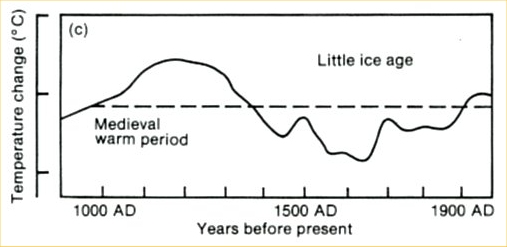
“Climate sceptics” do not like this and keep coming up with their own temperature histories. One of the weirdest has been circulated for years by German high-school teacher E.G. Beck (notorious for his equally weird CO2 curve). This history shows a medieval warm phase that is warmer than current climate by more than 1 ºC (see Figure 2). So how did Beck get this curve?
Fig. 2, modified from E.G. Beck (we added the green parts).
The curve is a fake in several respects. It originally is taken from the first IPCC report of 1990: a scan of the original is shown in Figure 3. At that time, no large-scale temperature reconstructions were available yet. To give an indication of past climate variability, the report showed Lamb’s Central England estimate. (Unfortunately this was not stated in the report – an oversight which shows that IPCC review procedures in the early days were not what they are now. We will post in more detail on the history of this curve another time.)
Fig. 3. The past millennium as shown in the first IPCC report of 1990, before quantitative large-scale reconstructions were available. This curve was based on Lamb’s estimated climate history for central England.
But Beck did not stop at simply using this outdated curve, he modified it as highlighted in green in Figure 2. First, he added a wrong temperature scale – the tick marks in the old IPCC report represent 1 ºC, so Beck’s claimed range of 5 ºC exaggerates the past temperature variations by more than a factor of three. Second, the original curve only goes up to the 1970’s. Since then, Northern Hemisphere temperatures have increased by about 0.6 ºC and those in central England even more – so whatever you take this curve for, if it were continued to present, the current temperature would be above the Medieval level, as in the proper reconstructions available today. As this would destroy his message, Beck applied another fakery: he extended the curve flat up to the year 2000, thereby denying the measured warming since the 1970s. With this trick, his curve looks as if it was warmer in Medieval times than now.
When approached directly about these issues, Beck published a modified curve on a website. He changed the temperature range from 5 ºC to 4.5 ºC – but he shortened the arrow as well, so this was just cosmetics. He also added instrumental temperatures for the 20th Century at the end – but with his wrong temperature scale, they are completely out of proportion. (In fact his version suggests temperatures have warmed by 2 ºC since 1900, more than twice of what is actually observed!)
Beck goes even further: in a recent article (in German), he has the audacity to claim that his manipulated curve is right and the more recent scientific results shown by IPCC are wrong. And for years, he has offered his curve on an internet site (biokurs.de) that distributes teaching materials for schools, with support from German school authorities. It is quite likely that his fake curve has been shown (and will continue to be shown) to many school children.


Blair Dowden (#448 wrote:
Maybe I am missing a few things here… Come to think of it, probably more than a few things – in fact I’m sure of it. But why do you think that the greenhouse effect is “more important” in the upper atmosphere?
The core principle behind climate sensitivity to CO2 levels is principally that CO2 has a longer duration of stay than water vapor (decades or centuries for CO2 compared to perhaps ten days for water vapor). At higher altitudes where water vapor is negligible, carbon dioxide is an effective greenhouse gas which re-emits enough infrared towards the lower layers of the atmosphere and the surface, initiating the amplification of its effects through water vapor evaporation and the positive feedback which ensues. Both are important, and water vapor in the lower atmosphere is actually the stronger of the two greenhouse gas components – but carbon dioxide is regarded as more important in the sense that it regulates the process as the result of its stay time in the atmosphere.
re 446, et al: “According to the Greenhouse Effect page in Wikipedia, “Most of the infrared absorption in the atmosphere can be thought of as occurring while two molecules are colliding. The absorption due to a photon interacting with a lone molecule is relatively small.” So there is not even a single specific molecule that receives the longwave radiation.
I know I’m behind here; sorry; but….. the above can not be true can it? A molecule only absorbs LW-IR at the instant it is colliding with another molecule???
Secondly, does a gas molecule radiate other than a re-emissiom of previously absorbed radiation? In other words does it emit based on its temperature in a Planck/blackbody (graybody??) fashion, also. If so, would this emitted energy come from molecular bonds (excited state) or kinetic energy (“relaxed state), the latter reducing its temperature? Would this emission tend to heat the surface (if not lost in space)? What wavelength does it emit? One molecule certainly can’t emit at a Planck distribution….. can it???
Re #442
Blair,
The models reproduce the current climate fairly well, but that does not mean that they are correct. It does mean that looking to see where they match reality is only going to confirm that they are correct. You have to look where they fail to match reality, if you are going to find evidence that they are wrong.
The paper you cite uses models based on the down-welling paradigm to calculate the DLF, so it would be surprising if its results were very different from that of the GCMs which use the same paradigm. Despite that, they seem to have found a difference of 30 W m-2 (~10%) in regions where there is very little water vapour and so the greenhouse effect of CO2 dominates. viz, deserts and at high altitude.
There is a balance between collisions and radiation in such a way that the CO2 radiates back to Earth, but it is not dependent on the temperature of the air. Rather it is due to the air pressure (i.e. no of collisions.) The radiation absorbed by CO2 is converted into heat of the air which convects, so when the CO2 molecule is excited again by collisions and re-emits, it is at a higher altitude where its radiation is absorbed before it can each the surface of the Earth.
Thanks for getting me to try to explain this, and my apologies for having done it so badly/hastily.
Once again, this issue of why adding CO2 to the atmosphere will warm the atmosphere, even though the infrared absorption of CO2 is saturated at ground level, was worked out well over fifty years ago. Just read through http://www.aip.org/history/climate/Radmath.htm for the history.
There are really three areas of climate science that skeptics, contrarians, denialists and other fossil fuel-funded PR types are trying to attack: the paleoclimate studies, the direct observations of climate change, and the climate models.
Many of these areas are indeed complex, but so are computers. One could come up with all kinds of ‘skeptical arguments’ about whether or not you can use electricity and semiconductors to store information, for example, that most people without degrees in physics couldn’t follow, such as “My belief is that quantum interference effects would lead to loss of information,” and so on.
The fact of the matter is that all three areas of climate science indicate that human use of fossil fuels and deforestation are leading to the highest rate of global warming ever measured, and that unless fossil fuels are replaced by renewable, carbon-neutral energy technologies the rate of global warming will continue to accelerate, with devastating consequences for people all over the planet.
Re #454: Ike Solem — How about carbon-negative instead?
http://www1.eere.energy.gov/biomass
Ike, I am not challenging the accuracy of the science that says carbon dioxide is responsible for 20% of the greenhouse effect, I am challenging the scientists to explain why that is the case. I believe that any subject can be explained at any level of complexity, what I am looking for is something beyond “its like a blanket that keeps the Earth warm.”
The basic facts are carbon dioxide levels are less than one tenth those of water vapor in the atmosphere, and water vapor absorbs a much wider spectrum of radiation. The simplistic conclusion is carbon dioxide is only a few percent of the greenhouse effect. The only way to get to 20% is for the upper atmosphere, where CO2 dominates, to be more important. On the basis of what I have learned so far in this thread, the greenhouse effect will be weaker because collisions of sufficient energy to cause a photon to be emitted will be less frequent.
I don’t actually believe this, but I don’t know why it is wrong. One explanation is that depends only on the temperature at which a greenhouse gas radiates into space, so colder gases at the top of the atmosphere (ie. carbon dioxide) will radiate at a lower temperature and the Earth will lose less energy. That sounds nice, but why is it so? It sounds like the greenhouse gas is being treated like a black body, which apparently is not the case.
Rod B, you are asking the same questions that I am, and I can hardly give you an authoritative answer based on something I learned this morning. I will point out the primary author of the Wikipedia article is Real Climate’s William Connolley.
[[The warmer the gas, the higher the probability that a collision will have sufficient energy to cause a photon to be emitted. So how is the greenhouse effect supposed to be more important in the upper atmosphere? I am still not understanding the end game here. ]]
That’s a result of radiation from the ground being mostly (though not near all) absorbed fairly low down. As a result, it’s hard to change the temperature of the lower levels directly. At upper levels the bands are less saturated and thus the greenhouse gases act as if they were a bit more sensitive. The mechanism on the atomic/molecular level is the same in each case, though.
[[I have a basic question/clarification (which may have already been discussed here, in which case I apologize). LW radiative energy is absorbed by GH gas molecules in bond translational or rotational energy, which does not raise that molecule’s temperature. Is this correct? Then the molecule will re-emit that radiation, losing its bond energy (in part); or it might collide with some other molecule. In this case does the molecular bond energy get transferred to the collidee’s bond energy (which can’t happen with N2 or O2… can it??) or as kinetic energy thereby increasing the temperature of the collidee molecule (and the atmosphere). Given either can happen, it sticks in my mind that the collision transfer is much less than the radiation emission…. or is it vice versa??? ]]
Well, you can’t really talk about the temperature of a single molecule; temperature is a statistical effect of the motion of large numbers of molecules. I don’t think absorption of a photon affects the molecule’s bond energy per se; what it does is kick an electron of one atom in that molecule to a higher, less stable level. The molecule will then lose energy either by radiating or by hitting another molecule. In either case, the amount gained or lost by the molecule in question should be identically the same, since we’re dealing with the quantum level of things and energy comes in discrete packets at that level. I’m probably off on some of the details here, but Gavin or somebody can correct me.
[[The basic facts are carbon dioxide levels are less than one tenth those of water vapor in the atmosphere, and water vapor absorbs a much wider spectrum of radiation. The simplistic conclusion is carbon dioxide is only a few percent of the greenhouse effect. The only way to get to 20% is for the upper atmosphere, where CO2 dominates, to be more important. On the basis of what I have learned so far in this thread, the greenhouse effect will be weaker because collisions of sufficient energy to cause a photon to be emitted will be less frequent.]]
Water vapor is indeed a stronger greenhouse gas, but it has a much shallower scale height than the rest of the atmosphere (2 km on average versus 8 km). CO2, on the other hand, is well mixed throughout the troposphere, so in most of the atmosphere it is the dominant absorber. Not enough to be the dominant absorber in the whole atmosphere, but enough to kick its contribution up to a higher level than you’d expect.
Blair Dowden (#456) wrote:
The problem, I suspect, lies not with your reasoning, but with the statement from Wikipedia. I don’t think that the following statement is wrong exactly, but it is ambiguous and lends itself to misinterpretation:
#452: “Most of the infrared absorption in the atmosphere can be thought of as occurring while two molecules are colliding. The absorption due to a photon interacting with a lone molecule is relatively small.”
Why does the author say “can be thought of”? Those words suggest that it is actually the individual molecules are doing the absorbing, but it also suggests that they are interacting and that the interactions are common enough that they affect the absorbtion and re-emission. As such, I believe that what is actually being refered to is the process by which some kinetic energy is lost or gained in between absorbtion and re-emission, that is, the process which results in the spreading of the spectal bands at higher pressures.
Two molecules can’t become excited together unless there is some sort of bond between them that can become knocked up into a higher state. But there aren’t any such bonds, otherwise they would be the same molecule – and the bands would be at different positions in the spectra. But they are at the same position, only spread out.
But I would go a little further. Photons may be interacting with the molecules even without collisions in between absorbtion and re-emission, but oftentimes the molecule will already be in an excited state such that it can’t be knocked up into that state. This is what happens when the center of the band becomes saturated. However, the wings of the band can become further saturated. This is what leads to the logarithmic response of the greenhouse gas as opposed to the earlier linear response when it is only the center of the band which becomes occupied at lower pressures. But this is also another reason why the author uses the words “can be thought of.”
In any case, the only quantum mechanics I have is what I managed to teach myself out of a couple of textbooks back in high school – and I never got to the application of it to greenhouse gases. So I could be wrong. But this is how I have managed to fit it together.
Blair, Tim, et al.,
Don’t get bogged down in all the quantum mechanics and math. You have a bunch of photons outbound from Earth’s surface and very few incoming intitially. Some of those photons get absorbed by greenhouse gas molecules. Roughly 50% of those that get absorbed are re-emitted toward space again, while the other 50% are re-emitted toward the ground. There will be some small fraction of excited molecules that relax via collision, but this is a small percentage for any given absorption. If such a collision does occur, the energetic molecule will rapidly thermalize due to collisions with other molecules. There will be repeated absorptions, re-emissions and collisions. However, in the end, one of two things will happen: 1)The photon makes it into space, escaping Earth, or 2)it gets re-absorbed and increases thermal energy of the air or of the surface. The more greenhouse molecules in the atmosphere, the more steps the molecule must take before it escapes, so if the probability of radiant energy becoming thermal energy at any given step is constant, then the greater the total probability that the photon increases the energy in the climatic sysetm. Now, as Earth warms, it emits more IR photons, so the probability that SOME of them escape increases, so eventually the system reaches a new equilibrium–unless the proportions of greenhouse gases also increase. Think of it as a random walk. Shorten the step size, and introduce a finite probability of getting hit by a car for every step you take, and the drunk never makes it across the street.
re 451 (Timothy): I have to revisit and re-question this again. (Maybe I’m just dense, as some have implied…[;-} )
First, I don’t see the “life” of water vapor being either meaningful or significant. If you are saying the average evaporated H2O molecule floats around for 10 days before precipitating out, I can understand that but have no idea why its relevant. A whole bunch of water evaporates over the tropics say, then later, often the same day, a whole bunch rains down. The water vapor stays between 2-4% day in day out with maybe a brief minute or hour during a storm when it drops to near zero. Is this not correct??
Second, this gets me back to the forcing/feedback thing. On average 86% of LW radiation is absorbed in the first couple hundred meters, about 65% by water vapor, 16-18% by CO2. Why is not H2O a direct and major forcing? Then when the 10-15% of the total gets to the stratosphere, a bunch gets absorbed by CO2 (none by H2O), re-emitted back down, and, assuming it gets past the high density lower troposphere, warms up the ocean some. Enough to significantly increasae the evaporation to allow significantly more absorption by water vapor to make it a significant positive feedback mechanism?????? More important than the forcing????? Sounds like a stretch…., but I’m asking.
Rod B (#462) wrote:
The effects of water are major – and they do result in the system amplifying a process which begins with and is maintained by carbon dioxide. Water vapor is responsible for much of the feedback by which more water is evaporated, although not its iniation. However, the system reaches a new equilibrium where the amount of water which falls as precipitation is equal to the amount of water which is evaporating, and since the time required for water to evaporate and precipitate is far shorter than the amount of time that carbon dioxide remains in the atmosphere, it is the amount of carbon dioxide which determines the level at which the equilibrium is achieved and maintained.
Enough, either directly or indirectly through absorbtion and re-emission which eventually reaches the surface that it is equivilent to a higher solar constant. In essence, this is part of what Ray Ladbury was explaining in #461 with regard to the subject of the random walk. The ultimate destination of a given photon will be either space or the ground, roughly fifty-fifty, although there is a slight preference for upwelling due to the fact that the legs in the upward direction will be slightly longer. To some extent this will no doubt be amplified as the result of multiple absorbtions and re-emissions, reducing the greenhouse effect, but not a great deal.
While I am still bogged down with the quantum mechanics, I would like to give my current view of the molecular collision issue raised by Tim in #460. Corrections are more than welcome.
A greenhouse gas molecule is only excited into a virbational state by a photon with the exact wavelength that kicks one of its electrons to a higher, less stable level (thanks, Barton). A collision with another molecule changes the state of the greenhouse gas molecule, which lets it absorb other wavelengths. This is why a collision is required, and this is what is behind the broadening of the absoprtion spectrum at higher atmospheric pressure.
Now a question: when the greenhouse gas molecule emits the photon after being energised by a collision, will it do this at the exact ideal wavelength, or the same range of wavelengths at which it could be energised by in the first place. I suspect the latter, but if the former is true then we have the wavelengths being “purified” by this process. This might help absorption higher in the atmosphere.
Leaving quantum mechanics behind, Ray gives a nice summary of the greenhouse effect in #461. One problem I have is the photon emissions seem to only warm the air and never reach the ground. The data, if I interpret it correctly, says a lot of downwelling longwave radiation reaches the ground, and the simple models I look at treat that as the only source of the warming. The other problem is it implies there is no difference in the upper troposphere. Without this carbon dioxide can only be responsible for a few percent of the greenhouse effect.
Barton deals with this in #457, but I don’t get the point. Why should the position of the molecule matter if each one has the same probability of absorbing the longwave radiation. In fact, I argued that there are less collisions and it is less likely a molecule will reach the energy level required to radiate, so the probability is even less. I hope someone explains the fault in my reasoning here.
Blair Dowden (#464) wrote:
I would assume that if energy is gained or lost as the result of collision with other molecules, it is what is left over and must be lost for it to drop into the ground state which is given up as the result of re-emission.
I think of it as a random ladder climb, where any given photon can climb up or down the ladder every time it is re-emitted. For any given photon, the path which it takes is either one leading to space or leading to the ground – no matter how many steps may be involved inbetween. I had said earlier that roughly half will end up escaping to space whereas roughly half reach the ground, but this is mistaken since with fewer steps in either direction, the photon will be more likely to take the direction of the fewer steps even if it initially heads off in the other direction.
The more carbon dioxide in the stratosphere or water vapor in the troposphere, the longer the walk, and the more energy which will remain within the atmosphere at any given time. The more energy in the atmosphere, the more energy in the form of photons that will reach the earth at any given time, raising its temperature. But even assuming that the radiation is re-absorbed by the ground after so many absorbtions and re-emissions in the atmosphere, it must ultimately escape to space if the temperature of both the ground and the atmosphere are to reach equilibrium. As such, it will pass through both the layers of water vapor and carbon dioxide. And where there is no overlap between the spectra of carbon dioxide and water vapor, photons re-emitted by carbon dioxide towards the ground will reach ground and show up in the spectra of the downwelling radiation.
If the center of the band is already saturated, that means that all of the molecules which could absorb photons and enter the excited state are already excited and are therefore incapable of reaching the excited state. Since there is more space, between molecules, there is less for the photons to collide with and therefore the carbon dioxide is more likely to be in a grounded state and capable of absorbing the photons in the center of the bands. Since water vapor is absent, this leaves carbon dioxide free to absorb the photons and re-emit them.
Collisions are just as likely to subtract energy as add it to the molecule which absorbs a photon. What is lost upon re-emission is what is necessary to reach the grounded state – or so I would think.
#464, vibrational transitions are changes in the movements of nuclei not electrons. Times associated with electronic transitions are fs, those with vibrational are ps and those with rotational are yet slower.
At atmospheric pressures, pressure broadening is associated with changes in the electric fields around the particular molecule that absorbs or emits due to the presence of another molecule within something like a micron or less.
Your question about the emission wavelengths is unclear to me. A molecule excited by a collision has some memory of it in its velocity (Doppler shift, something else we have not talked about).
Eli Rabett (#465) wrote:
That helps!
I should have seen it, but yes, I was thinking in terms of electrons when if we are talking of bending or rotating the molecule to achieve different states, it will be the nuclei, or to be more precise, the molecule itself which is knocked into an excited state.
Re #462 etc.
Rod,
The breakdown of the different greenhouse agents is based on this paper: Ramanathan and Coakley, Rev. Geophys and Space Phys., 16 465 (1978))http://www-ramanathan.ucsd.edu/publications/Ramanathan%20and%20Coakley%20RevGSP%201978.pdf
Basically they used models similar to Barton’s and run them with only one of each of the forcing agents and measured the effects. Then they can compare which causes the most effect.
But the big question being asked by scientists at present is how large will the rise in temperature be for a doubling of CO2. The reason they do not know is because as the temperature rises it will cause more water vapour to be created. It is the strength of this feedback which is unknown. Therefore they do not know the ratio of the effects of CO2 to H2O, the answer to your question. Nor do the scientists know the result of increasing CO2 because, its effect is amplified by H2O, but by how much is not known.
But they do know that increasing CO2 will increase temperature.
HTH,
Cheers, Alastair.
Re #464 Blair, your reasoning is OK. It is the models that are wrong. CO2 does not emit more radiation if it get warmer. So back radiation does not increase when the surface temperature rises.
There is quantum mechanical effect where its increase in radiation is frozen out. At the heart of the models that are discussed here is classical quantum theory. These quantum mechanical effects do not apply to stellar interiors, from which the models have been borrowed.
Fourier was not referring to a greenhouse when he explained why the world is as warm as it is. He was refferring to the work of Horace de Saussure and his hot box see: http://www.solarcooking.org/saussure.htm
Alastair McDonald (#469) wrote:
Sounds like it would be easy to design an experiment to test this. Probably Nobel prize material. I will look forward to reading the chapter on it when it makes its way into a textbook.
Eli,
A question. Timothy defines saturation as being when the molecules are already in their excited vibrational state. If so, would this not be a population inversion, and could you not get stimulated emission? And if it really isn’t a population inversion, then what is really meant by saturation?
Re #466: Eli, so the pressure broadening is associated with the presence of a nearby molecule, not with the collision itself. But is it correct that fixed quantum unit of energy will be gained when a photon is absorbed? And the same vibrational state can be acquired by a molecular collision with the right amount of energy?
Kirchoff’s law, if I understand it, says the greenhouse gas molecule will emit at the same wavelength at which the photon was absorbed. Pressure broadening increases the range of absorbing wavelengths, so I ams asking if the molecule will emit in the same wavelength range, or at the center of the range.
Blair and Alistair, the Wikipedia article helped jog my memory about the actual physics. Because the lifetime of the vibrational state is actually fairly long, the physical (especially electromagnetic) condition at the time of absorption and emission may be different. Therefore, there is no reason why the wavelengths of absorbed and emitted radiation will be the same. A molecule may absorb in the center of the line and emit in the tails depending on how many and what molecules/ions are present at the time of each process. You can’t think of the molecules in isolation.
http://en.wikipedia.org/wiki/Emission_line
[[The water vapor stays between 2-4% day in day out with maybe a brief minute or hour during a storm when it drops to near zero. Is this not correct??]]
Well, no. The time-averaged volume fraction of water vapor in the atmosphere is about 0.4%. It can get as high as 4% but it rarely does, except sometimes in the tropics.
[[Second, this gets me back to the forcing/feedback thing. On average 86% of LW radiation is absorbed in the first couple hundred meters, about 65% by water vapor, 16-18% by CO2. Why is not H2O a direct and major forcing?]]
Technically it is. But it’s not a problem for climate change because the fast turnover means it’s very, very hard to change the mean amount of water vapor in the air. If we artificially doubled it tomorrow, most of the excess would be gone in less than a month. So for climate change, CO2 is considered a forcing and H2O considered a feedback. It’s just easier to treat it that way mathematically, unless you’re writing the radiation code for a simulation.
ray ladbury (#473) wrote:
So the collisions are adding and subtracting energy between absorbtion and emission, and returning to ground state results in the emission of a photon carrying energy equal to whatever is required to return to ground state, I presume?
Fancy that. Actually I believe Alstair may have said as much earlier than anyone else, though.
[[CO2 does not emit more radiation if it get warmer. So back radiation does not increase when the surface temperature rises. ]]
This is wrong. If CO2 didn’t emit more radiation when it was hotter, then an increase in photons from the surface (hotter surface) would steadily heat the atmosphere more and more with time. If radiation doesn’t eliminate the excess energy, what does?
I think you’ve suggested that it must be conduction. You should be aware that conduction is much, much simpler and better understood than radiation. If conduction were doing it, the thermal conduction coefficients would all have to be wrong, and they were all determined in labs a long time ago.
Blair, you should be aware that Alastair’s ideas on radiation physics and the greenhouse effect are non-mainstream.
RE #441 Barton,
Sorry to have taken so long to get back to you.
Your model has not misled me. I already knew that the Manabe-Weatherald (M&W) scheme was faulty, when I asked you for your sources.
I think you are saying that it is invalid to average a line that absorbs totally in the first 100 m, say, with the gap between it and the next line and so get 50% absorption. This is correct at the top of the atmosphere, but if you then say that one third of the way up the atmosphere you will have absorbed 17% (1/3 of 50%) of the radiation, then that is wrong. At one third of the way up the atmosphere 50% of the radiation will be absorbed.
You argue that this all comes out in the wash, but that is due to another error in the M&W scheme. They introduced convection to the radiative-convective models. They implemented it by introducing a maximum lapse rate of 6.5 K km-1, the US Standard Atmosphere value, and convecting the layers when that value is exceeded. But since the atmosphere is being heated by the surface of the earth, convection is inevitable. Thus, by setting a maximum of 6.5 K km-1, they were setting the actual lapse rate to 6.5 K km-1. Thus the lapse rate inevitably matches the US Standard Atmosphere, no matter how the radiation scheme works. That is why, when Ray told you to increase the lapse rate to 9 K km-1 for Venus, your Venus model gave you the right answers there too.
This is not a criticism of the your version of the model. Your code was easy to understand and you implemented the algortithm in a simple manner. It is the algorithm that its wrong. I think you could prove that I am correct about that if you added a check to your code. Does the net outgoing long wave radiation from the surface of the earth equal the net amount radiated to space? If not, why is that energy not conserved?
Cheers, Alastair.
Barton Paul Levenson (#476) wrote:
True – but it is worthwhile to acknowledge the points that he makes which are “mainstream” – such as the loss or gain of energy by kinetic means between absorbtion and re-emission.
RE #476 Where Barton wrote “Blair, you should be aware that Alastair’s ideas on radiation physics and the greenhouse effect are non-mainstream.”
Yes, but my ideas make more sense :-)
Re #476 Where Barton wrote “If radiation doesn’t eliminate the excess energy, what does?”
And in #360 where Blair quoted Ray Pierrehumbert as writing
Planets only have one way of losing energy, which is by infrared radiation to space, often called “Outgoing Longwave Radiation,” or OLR.
Planets can cool in another way! By forming clouds they can reduce the incoming solar radiation to match the OLR. But clouds have second function because they radiate OLR from a high altitude where the greenhouse gases are less effective. However, they radiate as much heat downwards as they do upwards, with the result that when they cool the atmosphere they warm the Earth’s surface especially at night.
One way you can see this is with El Nino. When the seas in one of the hottest areas on the Earth – the Warm Pool – get too hot, clouds from there flow across the Pacific acting as a giant sunshade. It is not a change in OLR from the surface which produces the cooling, it is a reduction in incoming solar radiation.
No disrespect guys, but there are a lot of things that need to be discussed from this. I think I will put up a post on my blog tonight or tomorrow.
One important thing is that people are focusing on a particular CO2 molecule, watching it absorb and waiting for it to emit. Uh uh. Which molecule absorbs and which emits is essentially (that’s the picky scientist in me, I should write completely) random
Eli,
I’d welcome any clarification. It’s been a long time since I took a planetary atmospheres class.
Alistair–clouds do not really cool the planet in the sense that Ray Pierrehumbert is talking about. Rather, by blocking SW radiation, they keep it from warming. And the radiation from cloudtops is LWR, just what he was talking about.
Eli, I think I have now got the point that the CO2 molecule that absorbs the longwave radiation is not special. The air warms, and another CO2 molecule emits. This is very different from what I thought before.
Let me now propose what happens in the upper atmosphere. Only longwave radiation with higher intensity can excite the cold CO2 molecules. This means all the low intensity radiation escapes, but half of the higher intensity radiation is “reflected” back down. This process lowers the average intensity of the radiation that goes into space. This explains why a colder molecule appears to radiate less, without invoking Stefan-Boltzmann for a non-blackbody.
The “pinball” model of greenhouse warming is every time a photon is absorbed (by a greenhouse gas or the ground), the air gets a little warmer. More greenhouse gas means the photon bounces around a little more, and there is a little more warming.
The radiation balance model seems to say that none of that matters. Energy that is absorbed is radiated away again. The only thing that matters is how much energy escapes into space. If less energy goes into space the Earth will warm to compensate. The carbon dioxide high in the atmosphere reduces the intensity of what is radiated into space as I described above, so that is why it is relatively important.
Lets see this one get shot down.
Blair,
Even under with the current models you are way off track. You wrote:
Eli, I think I have now got the point that the CO2 molecule that absorbs the longwave radiation is not special. The air warms, and another CO2 molecule emits. This is very different from what I thought before.
The CO2 molecules are special. Only CO2 and H2O molecules absorb and emit infrared (IR) radiation, so CO2 molecules are special.
The air cannot warm and another molecule emit, because that violates the the Law of Conservation of Energy. I may seem like splitting hairs, but for another molecule to emit then the air must then cool again. In such a scenario the air does not warm because all the radiation absorbed is re-emitted.
You then wrote: Let me now propose what happens in the upper atmosphere. Only longwave radiation with higher intensity can excite the cold CO2 molecules. This means all the low intensity radiation escapes, but half of the higher intensity radiation is “reflected” back down.
The intensity of the radiation is irrelevant. It is the frequency of the radiation which excites CO2 molecules. At the top of the atmosphere (TOA) the molecules are radiating to space but in the layer below the molecules are radiating upwards so the TOA molecules are always being refreshed. In other words, radiation from the TOA back down is equalised by radiation upwards.
HTH,
Cheers, Alastair
Ray,
Prof. Ray Pierrehumbert believes that the only way to cool the Earth is to emit more OLR. What I am saying is that clouds can also cool the Earth by reflecting incoming shortwave radiation (ISR)
Re #484: Alastair, I thought water vapor was unique in that longwave radiation is transformed into rotation. Other than that, all greenhouse gases absorb and emit radiation. Is that not what defines what is a greenhouse gas?
Translating kinetic molecular energy into vibrational energy, then emitting does not violate conservation of energy as long as the emission does not lose more energy than was added to the system by the original absorption. For example, it might take two absorptions to add enough energy to generate one emission.
Eli in #291 says “the absorption per molecule at line center is HIGHER for colder molecules“. Maybe I am misinterpreting this statement.
Re #486 Blair,
AFAIK, the fact that water vapor is rotationally IR active and carbon dioxide is not, does not have much bearing on the greenhouse effect. It only affects which parts of the spectrum are absorbed and emitted.
As I understand it, both gases are vibrationally IR active, and will absorb and emit on lines which are a combinations of vibrational and rotational modes. The original equipment used to investigate the absorption was not precise enough to separate out the lines, and it was believed that the absorption was occurring in bands. The term band is still used for groups of lines.
But I think I will wait to see what Eli writes before I say any more :-(.
re 474: [Well, no. The time-averaged volume fraction of water vapor in the atmosphere is about 0.4%. It can get as high as 4% but it rarely does, except sometimes in the tropics.]
always had trouble with decimal points; but what’s a couple of points between friends??? Btw, shouldn’t it be 0.04%???
Alastair, your arguments about reversibility are not valid when you talk about processes involving a single molecule–the laws governing absorption and emission and collision between 2 molecules are time-reversal invariant. Arguments appealing to entropy only work on assemblies of particles above a certain size.
In any case, I’ve come to realize that at some level, the physics isn’t adequately described by considering a single molecule in isolation. Just as the periodic structure of atoms in a solid turns energy lines into bands, so the surrounding molecules also affect the absorption and emission of IR photons–and vice versa. The continual fluctuations of gas densities ensure that both emission and absorption lines will be significantly broadened and that at some level energy will be shared with the gas. Certainly, the ultimate fate of the photons is to either radiate away or to warm the surface, water or air, and the latter is much more probable as the number of steps to free space increases.
Very helpful discussion for me. I still have a couple of clarifications/questions (some repeated). I thought the IR absorbed by a GHG added energy to molecular bonds (and maybe nucleon bonds???), translational or rotational, or to electron energy states, and this does NOT increase the temperature of the molecule which is a function only of its kinetic energy. Is this correct?
Secondly, can that energized molecule release its energy (to get back to its relaxed state) by re-emitting IR radiation AND/OR colliding with another molecule (greenhouse gas type or not) and transferring/transforming its molecular energy to kinetic energy (and higher temp) in the collidee?
Finally, can molecule #2 which now has increased kinetic energy release some of that as radiation (I guess in a black/graybody fashion…), and at what wavelength? Or is it only transferrable as kinetic energy, sharing its temp with other molecules of the greenhouse type or not.
On a different note: re 463 — Timothy, do you mean like “In The Beginning……” when the earth was cold (??) and there was no/little water vapor, that the CO2 triggered the warming and eventual (and increasing) evaporation? What would happen to today’s water vapor and its GH effect if all of the CO2 was taken away? (I need to check out Alastair’s link in 468.) Also, I still don’t get the relevance of the average lifetime of a H2O molecule compared to CO2 and how that effects forcing vs. feedback. Or is it like Barton says in 474 (if i got it) that H2O is really (mostly) a forcing but its different odd process of evaporating and precipitating (and probably aggravated by the latent heat stuff) makes it look like a feedback mechanism — at least it’s easier to handle mathematically in models as a feedback.
Rod, I’ll try here. Again, the issue is that the molecule does not exist in isolation. The surrounding molecules create a perturbation that alters the electromagnetic field in which the electrons orbit. Now if the surrounding gas acts on the atoms, then by definition, the atom must also act on the surrounding gas, so there has to be at least some sharing of energy.
Kinetic energy only gets converted into some other kind of energy by collision–or indirectly by perturbing other atoms. Also, remember that every photon has momentum, so there are small kicks every time a photon is absorbed or emitted.
People here may want to look at
http://en.wikipedia.org/wiki/Spontaneous_emission
The discussion is sort-of semi-conductor centric. Obviously the atmosphere does not have a crystal lattice structure etc…but the article and the links may never-the-less be helpful.
Also..for those that would contend there is something wrong with the general understanding of how IR radiation interacts with gassed (CO2 in particular). I would counter that we have understood this well enough to build lasers based on CO2 for decades now. (see http://www.laserk.com/newsletters/whiteTHE.html)
[[always had trouble with decimal points; but what’s a couple of points between friends??? Btw, shouldn’t it be 0.04%??? ]]
No. That would be about the concentration of carbon dioxide. Water vapor averages about ten times higher.
Re: Alastair McDonald:
I agree with most, maybe all of what you have said but I havn’t read it all.
I say, CO2 and CH4 are not greenhouse gases because there is no continuum absorption band for them to exchange energy, therefore no radiative equilibrium, as is the case for water.
Stated differently, CO2 and CH4 are not gray (therefore greenhouse) gases; they are white gases that exchange particular energies. Climate models (all as far as I can find out)which use wavelength bands in the gray gas approximation will not accurately model their properties, particularly the concentration dependence.
re: 493 Donovan
Planck specifically excluded flourescence — spontantous emission — from his theory. Have you found a description of what is implemented in the climate models that is more specific than “grey body”?
Allan Ames (#496) wrote:
They have been taking into account the specific absorption bands for various greenhouse gases for quite some time now – at least as far back as the 1980s. Typically, when they refer to this aspect of the calculations, they will be refering to “radiation codes.” The results of these calculations will then be compared against actual upwelling and downwelling radiation.
PS
Re: Radiation Calculations (Allan Ames 495, 496)
Anyway, you might want to check the detailed comment by Eli Rabett regarding the comparisons of projected spectra and actual spectra at #180
The Weirdest millenium
Eli Rabett â?? 3 Jun 2007
https://www.realclimate.org/index.php?p=450#comment-34296
Or for how this fits in to the modeling, check:
Stoat: How (coupled AO) GCMs work
2005-11-03
http://mustelid.blogspot.com/2005/11/how-coupled-ao-gcms-work.html
[[I say, CO2 and CH4 are not greenhouse gases because there is no continuum absorption band for them to exchange energy, therefore no radiative equilibrium, as is the case for water.
Stated differently, CO2 and CH4 are not gray (therefore greenhouse) gases; they are white gases that exchange particular energies. Climate models (all as far as I can find out)which use wavelength bands in the gray gas approximation will not accurately model their properties, particularly the concentration dependence. ]]
What in the world is this supposed to mean? CO2 has “no radiative equilibrium?” Radiative equilibrium isn’t something that applies to an element, it applies to a material body.
A greenhouse gas does not have to be a gray radiator to absorb and emit energy. That’s just wrong. Even with water vapor, the vast majority of its absorption of infrared light happens at the various absorption bands, not in the continuum, which has a very small absorption coefficient.
re 498 Timothy Chase
There are dozens of radiation codes, all different in some way. GCMs start with Schuster�Schwarzschild & Stefan-Boltzmann and work backward toward line by line. The issue is exactly how the parameterization handles additional molecules relative to the qualification tests. I question it is done correctly because I do not find the output reasonable.
Interstingly, even SS-SB might work somewhat with CF2Cl2 because of the large dipole moment, which is what I think makes water so special.